Transcatheter perimembranous ventricular septal defect closure under transthoracic echocardiographic guidance without fluoroscopy
Introduction
Ventricular septal defect (VSD), which in 70% of cases is perimembranous, is the most common congenital cardiac malformation, accounting for approximately 30% of cases of congenital heart disease (1). Open-heart repair with median sternotomy is the mainstream therapy for VSD, while minimally invasive partial sternotomy and right minithoracotomy were used to decrease the risk of injury induced by surgery. However, changes in location or length of the incision did not change the essence of the open-heart procedure (e.g., the need of cardiopulmonary bypass). Since hybrid procedures integrating interventional and surgical technology were used in different fields of cardiovascular diseases, cardiovascular surgeons invented transthoracic device closure to treat simple congenital heart diseases, such as arterial septal defect (ASD) and VSD, under echocardiographic guidance. This approach was inspired by the percutaneous device closure technology. Several studies reported mid- and long-term results of these procedures; the results showed comparable outcomes with those of cardiopulmonary bypass operation in different centers from China (2-5). Fuwai Hospital organized hybrid teams including cardiac surgeons, pediatricians, echocardiographers, and interventional cardiologists to explore new technologies, especially hybrid procedures that selected patients may benefit from. After the safety and efficacy of transthoracic device closure for perimembranous VSD (pm-VSD) were confirmed in our center (6,7), and the learning curve of transcatheter procedures under echocardiographic guidance alone was overcome by preforming device closure for ASD and patent ductus arteriosus (PDA) (8,9), percutaneous pm-VSD closure under TTE guidance alone was finally attempted in our center. In the present study, we described detailed methods of percutaneous pm-VSD closure via the femoral artery using TTE as the sole imaging tool and reported the mid-term results of this procedure.
Methods
Patients
Patients with pm-VSD were selected to attempt device closure under TTE guidance alone if preoperative TTE assessment revealed that the distance between the rim of the VSD and the aortic annulus was more than 2 mm. The diameter of the VSD should be within the range of 3 to 8 mm. The patients should be older than 1 year and have a body weight of more than 10 kg. Patients with aortic valve prolapse or more than trivial regurgitation, infectious endocarditis, severe pulmonary artery hypertension with a right-to-left shunt, and cardiac malformations necessitating treatment under cardiopulmonary bypass were excluded.
Informed consent was obtained from all patients or their legal guardians prior to inclusion in this study. The Ethics Committee of Fuwai Hospital approved this retrospective analysis (approval No. 2015-707).
Device description
The devices used in this study were self-expending concentric occluders with two disks and a connecting waist, made of 0.04 mm nitinol wire (Starway Medical Technology Inc., Beijing, China) (Figure 1). The waist diameters of the occluders ranged from 4 to 10 mm with 1 mm increment. The length of the waist was 5 mm in different sizes. Each disk was 4 mm larger than the connecting waist.
Procedures (Figures 2-9)
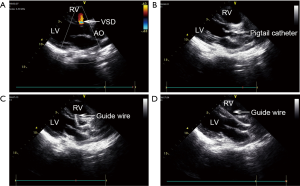
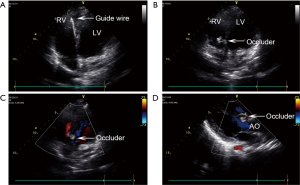






The procedure was performed in an ordinary operation room. Preoperative TTE was performed to identify the VSD position and measure its diameter (Figure 2A). With patient in a supine position, heparin (100 IU/kg) was administered and the right femoral artery was punctured under local anesthesia or sedation using propofol with spontaneously breathing to insert a 5-Fr arterial sheath. A 5-Fr pigtail catheter was then trimmed according to the VSD direction to facilitate a circle formation of approximately 1/3 by its head. The 5-Fr pigtail catheter and guide wire were introduced via the arterial sheath. Under the long axis view of the aorta guidance, the guide wire and catheter were inserted to the ascending aorta and into the left ventricle through the aortic valve (Figure 4). The catheter was then adjusted to make its tip face the VSD (Figures 2B,5), and the guide wire was gently advanced through the VSD into the right ventricle (Figures 2C,D,6). Finding the guide wire in the right ventricle on the TTE four-chamber view confirmed that the guide wire had passed through the VSD (Figure 3A). The occluder was chosen with a 2 mm larger waist than the VSD diameter measured by TTE before the procedure, and the corresponding delivery system was selected. The pigtail catheter was then exchanged for the delivery sheath under echocardiographic monitoring. Upon removal of the guide wire, the occluder was inserted through the delivery sheath. Under echocardiographic monitoring, the right ventricular side disc of the occluder was released (Figure 3B). With the occluder disc covering the right ventricular side of the VSD, the delivery cable was fixed, the delivery sheath was retrieved, and the left ventricular side disc was released (Figure 7). Upon echocardiographic confirmation of correct occluder positioning and shape, the absence of significant residual shunt and no touch between the occluder and aortic valve (Figure 3C,D), the occluder was released by rotating the cable anticlockwise under TTE guidance (Figure 8), and delivery and arterial sheaths were removed. Subsequently, the occluder positioning and shape, residual shunt and aortic regurgitation were examined again (Figure 9). The wound was routinely compressed and bandaged after hemostasis was achieved. Outpatient follow-up was conducted at 1, 3, 6, and 12 months after the procedure and yearly thereafter.
According to the experience at our center, several factors can improve technology safety and expedite the learning curve. First, this technique requires an experienced team including surgeons, cardiologist, echocardiographers, and anesthesiologist. The team should be able to perform open-heart surgery in case of an emergency to ensure patient’s safety. Second, the working distance should be carefully marked. We measured the distance between the left second intercostal parasternal space and the right femoral artery puncture site as the working distance before the procedure, and then marked the corresponding distance on the catheter. When the catheter was inserted into the body and reached the measured distance, we rotated the catheter to facilitate the detection of its position in the ascending aorta. Third, accurate positioning must be ensured. As the pigtail catheter is being withdrawn from the left ventricle, its insertion depth should be marked. The delivery sheath should be inserted 2–4 cm beyond the pigtail catheter’s insertion depth, and just reach into the right ventricle through the VSD without advancing too far. Finally, only patients with a VSD located more than 2 mm from the aortic edge should be selected for this procedure, because the delivery path restricts the use of an asymmetric occluder.
Statistical analysis
SPSS 20.0 software package (IBM Corp., Armonk, NY, USA) was used for statistical analysis. Data were expressed as mean ± standard deviation.
Results
Patients characteristics
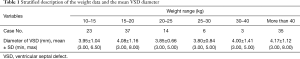
Between October 2012 and July 2016, pm-VSD closure under TTE guidance alone was attempted in 118 patients (51 males, 67 females). The mean age was 11.7±12.5 years (range, 1.0–53.0 years) and the mean body weight was 32.2±21.6 kg (range, 11.5–102.0 kg). The mean diameter of the VSD was 4.0±1.1 mm (range, 3.0–8.0 mm). Stratified description of the weight data and the mean VSD diameter of each group were supplied in Table 1. Preoperative TTE assessment revealed that 12 patients had mild or less tricuspid regurgitation, one patient had moderate tricuspid regurgitation, and two patients had trivial aortic regurgitation.
Full table
Perioperative results
The VSD was successfully closed using the occluders under TTE guidance alone in 111 patents (94%). The average procedural time from the femoral artery puncture to sheath removal was 44.9±7.3 minutes (range, 29.0–65.0 minutes). The following sizes of devices were used: size 4 (n=7), size 5 (n=34), size 6 (n=40), size 7 (n=16), size 8 (n=8), size 9 (n=4), and size 10 (n=2). Nine patients had a residual shunt smaller than 2 mm immediately after the procedure. The residual shunt disappeared spontaneously in five patients before discharge. None of them had hemolysis after the procedure. New-onset mild or less tricuspid regurgitation was observed in five patients and trivial aortic regurgitation was observed in two patients. Tricuspid or aortic regurgitation existing before the procedure was not improved or aggravated obviously after the procedure. Incomplete and complete RBBB occurred in seven and two patients. Among them, three patients with incomplete RBBB and one patient with complete RBBB reverted to a normal sinus rhythm before discharge. No patient developed complete atrioventricular block (cAVB) before discharge.
Pm-VSD closure by transcatheter occluder implantation under TTE guidance alone failed in seven patients. Conversion to perventricular closure via transthoracic small incision under transesophageal echocardiographic guidance was attempted in three patients because of compression of left disc to aortic valve leading to more than moderate regurgitation. Two patients received occluder removal and surgical repair was performed because of immediate appearance of cAVB after the device implantation. They reverted to normal sinus rhythm after occluder removal. In addition, conversion to surgical repair was necessary in other two patients because of an immediate residual shunt larger than 2 mm.
The mean hospitalization time was 3.7±1.3 days, and all patients were successfully discharged from the hospital. No other complications were observed before the discharge.
Follow-up results
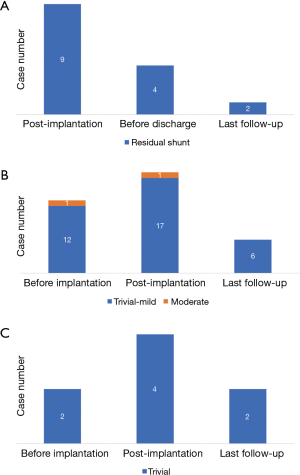
The mean follow-up time was 3.4±2.3 years. During the follow-up, the residual shunt completely closed within 3 months after the procedure in most patients who underwent pm-VSD closure. Only two patients presented residual shunt at their last follow-up (Figure 10A). Except for five patients who continued to experience RBBB after the discharge, six patients developed new-onset RBBB including one with complete RBBB. Progression form incomplete to complete RBBB was not observed in any of them. At the last follow-up, seven patients had RBBB including one with complete RBBB. However, none of them presented any sign of atrioventricular conduction delay. Additionally, no patient had cAVB during the follow-up. At the last follow-up, the degree of tricuspid regurgitation was improved, and only six patients had mild or less regurgitation (3 of them had tricuspid regurgitation before the procedure, including 1 with moderate regurgitation) (Figure 10B). Meanwhile, two patients still had trivial aortic regurgitation (1 of them had new-onset regurgitation after the procedure and another patient already had it before the procedure). However, no aggravation was noted during the follow-up (Figure 10C). Other complications, such as pericardial effusion and occluder malposition, were not observed.
Discussion
Our initial single center experience suggests that percutaneous pm-VSD closure under TTE guidance alone is feasible, safe, and effective. Echocardiography has the advantages of displaying valve function and hemodynamics, and may facilitate the process of device closure by assessment of the relationship between the occluder and relevant valve, and residual shunt. Therefore, echocardiography may play a critical role in the device closure procedure. Transesophageal echocardiography (TEE) as the only guidance for transcatheter closure of ASD using Amplatzer occluder was first reported in 2000 (16). Echocardiography was considered an alternative tool offering high-quality real-time anatomical image to provide guidance for cardiac structural interventions (17). However, TEE use may encounter several disadvantages, including the increase of anesthetic dose, possibility of mechanical intubation, and oropharyngeal impairment. A prospective randomized trail reported in 2013 concluded that TTE was as efficacious and safe as TEE for assessment and guidance of ASD in selected patients (18). Subsequently, TTE use as the guidance for device closure procedure increased (19,20). Transcatheter ASD closure, PDA closure, and balloon pulmonary valvuloplasty under TTE guidance without fluoroscopy were performed in our center with good early and mid-term results (8,9,21), creating a methodology of transcatheter procedure under echocardiographic guidance without fluoroscopy that was used in the present study. Transcatheter pm-VSD closure has more challenges than ASD or PDA closure in terms of technical details, such as retrograde operation and aortic valve assessment. Additionally, there are tremendous differences between echocardiographic and fluoroscopic guidance. Therefore, it is recommended that transcatheter procedure under echocardiographic guidance should start with ASD or PDA closure, which may help to overcome the learning curve and keep patients safe. In the present study, the procedure time from the femoral artery puncture to sheath removal was 44.9±7.3 minutes (range, 29.0–65.0 minutes),and was shorter in later cases than in the initial cases, suggesting that an experienced team would facilitate this procedure. The reported mean procedure time of transcatheter pm-VSD closure under fluoroscopic guidance varies between 44 and 122 minutes (22-25), and seems to be somewhat longer than that in the present study. Without regard for different occluder systems, the procedure time in our series indicated that the feasibility of device implantation under TTE guidance was not worse than that reported in other series.
Cardiac conduction block is the most concerned complication of transcatheter pm-VSD closure, especially cAVB is so feared that this therapy was abandoned in many institutions. Atrioventricular block may occur immediately due to squeezing effect of the device discs placement, or later due to device-induced inflammation and fibrous tissue formation (26). The reported incidence of cAVB ranges between 0% and 5.7% in different studies (23,27-29). A systematic review reported that the pooled estimate for cAVB was 2.7% [95% confidence interval (CI): 1.6–3.2%] with higher rates in smaller children. In our series, two patients had cAVB immediately after the device implantation and both reverted to normal sinus rhythm after the device removal. Both patients had a weight just over 10 kg, but needed an occluder of size 8 due to a relatively big VSD. It seems that immediate or early onset of cAVB can occur more frequently in patients with lower weight and larger VSD. Late-onset cAVB in patients with device implantation was not observed during follow-up. This may be attributed to the occluders used in our series, which were very soft devices with a longer waist (5 mm) that reduces the radial and clamping force. Recently, the Amplatzer membranous VSD occluder 2 and Nit-Occlud®Lê VSD coil was invented to reduce the risk of cAVB by decreasing radial and clamping forces in different designs. Tzikas et al. reported mid-term results of pm-VSD closure with an Amplatzer membranous VSD occluder 2 in 18 patients. No cAVB occurred during the follow-up of 14±3 months (25). Nikolaus et al. reported one case of transient cAVB, but no permanent cAVB in 102 patients with Nit-Occlud®Lê VSD coil implantation. Additionally, the off-label use of the Amplatzer Duct-Occluder II for closure of pm-VSD was reported (30-33). In these studies, only two cases developed cAVB and required occluder removal. RBBB is another common conduction abnormality other than cAVB, with an incidence ranging from 0% to 5.7% (23,27-29). Our results are comparable with those of previous studies, as 7/111 patients with new-onset RBBB were detected; however, no left bundle branch block or other arrhythmias were detected.
The residual shunt is the most common complication in VSD device closure. According to a review of 35 studies involving 4,138 cases, the pooled estimate of transient shunt was 25.5% (95% CI: 18.9–32.1%) and the pooled estimate of permanent shunt was 3.1% (95% CI: 2.0–4.1%) (27). In the present study, the rate of immediate post-procedural residual shunt was 8.1% and only two patients presented a residual shunt at the last follow-up. These patients were followed-up closely without signs of hemolysis.
Tricuspid and aortic regurgitation are also among the main complications associated with pm-VSD device closure. Yang et al. reported that secondary impairments of the tricuspid and aortic valve seem to be common, with a pooled estimate of a rate of 4.9% (95% CI: 3.4–6.4%). The rate of permanent valvular defect was 2.3% (95% CI: 1.3–3.3%), with tricuspid regurgitation in 1.7% (95% CI: 0.8–2.5%) and aortic regurgitation in 2.0% (95% CI: 1.0–2.9%) of cases (27). In the present series, only six patients had mild or less tricuspid regurgitation and two patients had trivial regurgitation finally. Some degree of aortic or tricuspid regurgitation was present before the procedure, secondary to the pm-VSD. Elimination of this defect can lessen valve regurgitation, particularly the tricuspid regurgitation. The improvement from trivial to none or from moderate to mild tricuspid regurgitation was observed in ten patients of our series. These results suggest the advantages of echocardiographic guidance in preventing valve complications. Pm-VSD device closure was performed under TTE guidance without an arteriovenous circuit, thereby avoiding the tricuspid valve damage. In addition, echocardiography might minimize the chance of tricuspid regurgitation by providing real-time display of the course of the right-side disc covering the right ventricular side defect and its relationship with the tricuspid valve and chordae tendineae. In terms of aortic regurgitation, it is important to ensure a minimal distance of 2 mm between VSD and the aortic valve and absence of aortic valve prolapse or more than trivial regurgitation before the procedure. Before the occluder release, Doppler assessment of the aortic regurgitation was not reliable to judge the position of the occluder to the aortic valve. Instead, B-Mode ultrasonography was used to confirm no touch between the occluder and the aortic valve.
Study limitations
This study was limited to some extent because it was a retrospective study, which only evaluated the results of patients that met the inclusion criteria. The largest VSD diameter was 8 mm; whether the procedure is suitable for larger defects remains to be determined. In addition, we only used one type of occluder and all used occluders were concentric. Other occluder systems were not attempted and the usability under TTE guidance alone should be verified. Finally, the sample size and the follow-up period may be judged to be only moderate. Therefore, long-term performance remains to be investigated.
Conclusions
The present study showed that percutaneous pm-VSD closure can be successfully performed under TTE guidance alone in selected patients with weight more than 10kg and VSD smaller than 8 mm. The incidence of complications using this new method is similar to that reported by studies that used fluoroscopic guidance. Larger, multicenter, and prospective controlled studies are necessary to further evaluate the feasibility and long-term results of this method.
Acknowledgements
Funding: This study was supported by 13th 5-year Key National R & D Program [No. 2016YFC1302004] and CAMS Innovation Fund for Medical Sciences [No. 2017-I2M-4-001].
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The Ethics Committee of Fuwai Hospital approved this retrospective analysis (approval No.:2015-707). Informed consent was obtained from all patients or their legal guardians prior to inclusion in this study.
References
- Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol 2002;39:1890-900. [Crossref] [PubMed]
- Xing Q, Pan S, An Q, et al. Minimally invasive perventricular device closure of perimembranous ventricular septal defect without cardiopulmonary bypass: multicenter experience and mid-term follow-up. J Thorac Cardiovasc Surg 2010;139:1409-15. [Crossref] [PubMed]
- Xing Q, Wu Q, Shi L, et al. Minimally invasive transthoracic device closure of isolated ventricular septal defects without cardiopulmonary bypass: long-term follow-up results. J Thorac Cardiovasc Surg 2015;149:257-64. [Crossref] [PubMed]
- Wang S, Zhuang Z, Zhang H, et al. Perventricular closure of perimembranous ventricular septal defects using the concentric occluder device. Pediatr Cardiol 2014;35:580-6. [Crossref] [PubMed]
- Song S, Fan T, Li B, et al. Minimally Invasive Peratrial Device Closure of Perimembranous Ventricular Septal Defect Through a Right Infraaxillary Route: Clinical Experience and Preliminary Results. Ann Thorac Surg 2017;103:199-204. [Crossref] [PubMed]
- Ou-Yang WB, Li SJ, Wang SZ, et al. Echocardiographic Guided Closure of Perimembranous Ventricular Septal Defects. Ann Thorac Surg 2015;100:1398-402. [Crossref] [PubMed]
- Ou-Yang WB, Wang SZ, Hu SS, et al. Perventricular device closure of perimembranous ventricular septal defect: effectiveness of symmetric and asymmetric occluders. Eur J Cardiothorac Surg 2017;51:478-82. [PubMed]
- Pan XB, Ou-Yang WB, Pang KJ, et al. Percutaneous Closure of Atrial Septal Defects Under Transthoracic Echocardiography Guidance Without Fluoroscopy or Intubation in Children. J Interv Cardiol 2015;28:390-5. [Crossref] [PubMed]
- Pan XB, Ouyang WB, Wang SZ, et al. Transthoracic Echocardiography-Guided Percutaneous Patent Ductus Arteriosus Occlusion: A New Strategy for Interventional Treatment. Echocardiography 2016;33:1040-5. [Crossref] [PubMed]
- Wang S, Ouyang W, Liu Y, et al. The trimmed pigtail catheter was inserted into the left ventricle through the aortic valve. Asvide 2018;5:776. Available online: http://www.asvide.com/article/view/27393
- Wang S, Ouyang W, Liu Y, et al. The trimmed pigtail catheter was adjusted to make its tip face the ventricular septal defect (VSD). Asvide 2018;5:777. Available online: http://www.asvide.com/article/view/27394
- Wang S, Ouyang W, Liu Y, et al. The guide wire was gently advanced through the ventricular septal defect (VSD) into the right ventricle. Asvide 2018;5:778. Available online: http://www.asvide.com/article/view/27395
- Wang S, Ouyang W, Liu Y, et al. The left ventricular side disc was released. Asvide 2018;5:779. Available online: http://www.asvide.com/article/view/27396
- Wang S, Ouyang W, Liu Y, et al. B-mode was used to assess the relationship between the occluder and aortic valve, before releasement. Asvide 2018;5:780. Available online: http://www.asvide.com/article/view/27397
- Wang S, Ouyang W, Liu Y, et al. The occluder positioning and shape, residual shunt and aortic regurgitation were examined again after releasement. Asvide 2018;5:781. Available online: http://www.asvide.com/article/view/27399
- Ewert P, Berger F, Daehnert I, et al. Transcatheter closure of atrial septal defects without fluoroscopy: feasibility of a new method. Circulation 2000;101:847-9. [Crossref] [PubMed]
- Mazic U, Gavora P, Masura J. The role of transesophageal echocardiography in transcatheter closure of secundum atrial septal defects by the Amplatzer septal occluder. Am Heart J 2001;142:482-8. [Crossref] [PubMed]
- Bartakian S, El-Said HG, Printz B, et al. Prospective randomized trial of transthoracic echocardiography versus transesophageal echocardiography for assessment and guidance of transcatheter closure of atrial septal defects in children using the Amplatzer septal occluder. JACC Cardiovasc Interv 2013;6:974-80. [Crossref] [PubMed]
- Sadiq N, Ullah M, Sultan M, et al. Transthoracic echocardiography as a measuring and guiding tool for transcatheter device closure of secundum atrial septal defect in young children. J Invasive Cardiol 2014;26:245-8. [PubMed]
- Chen W, Yan X, Huang Y, et al. Transthoracic echocardiography as an alternative major guidance to angiography during transcatheter closure of patent ductus arteriosus: technical feasibility and clinical relevance. Pediatr Cardiol 2015;36:14-9. [Crossref] [PubMed]
- Wang SZ, Ou-Yang WB, Hu SS, et al. First-in-Human Percutaneous Balloon Pulmonary Valvuloplasty Under Echocardiographic Guidance Only. Congenit Heart Dis 2016;11:716-20. [Crossref] [PubMed]
- Wang J, Zuo J, Yu S, et al. Effectiveness and Safety of Transcatheter Closure of Perimembranous Ventricular Septal Defects in Adults. Am J Cardiol 2016;117:980-7. [Crossref] [PubMed]
- Odemis E, Saygi M, Guzeltas A, et al. Transcatheter closure of perimembranous ventricular septal defects using Nit-Occlud((R)) Le VSD coil: early and mid-term results. Pediatr Cardiol 2014;35:817-23. [Crossref] [PubMed]
- Haas NA, Kock L, Bertram H, et al. Interventional VSD-Closure with the Nit-Occlud(R) Le VSD-Coil in 110 Patients: Early and Midterm Results of the EUREVECO-Registry. Pediatr Cardiol 2017;38:215-27. [Crossref] [PubMed]
- Tzikas A, Ibrahim R, Velasco-Sanchez D, et al. Transcatheter closure of perimembranous ventricular septal defect with the Amplatzer((R)) membranous VSD occluder 2: initial world experience and one-year follow-up. Catheter Cardiovasc Interv 2014;83:571-80. [Crossref] [PubMed]
- Ghaderian M, Merajie M, Mortezaeian H, et al. Efficacy and Safety of Using Amplatzer Ductal Occluder for Transcatheter Closure of Perimembranous Ventricular Septal Defect in Pediatrics. Iran J Pediatr 2015;25. [Crossref] [PubMed]
- Yang L, Tai BC, Khin LW, et al. A systematic review on the efficacy and safety of transcatheter device closure of ventricular septal defects (VSD). J Interv Cardiol 2014;27:260-72. [Crossref] [PubMed]
- Chen ZY, Lin BR, Chen WH, et al. Percutaneous device occlusion and minimally invasive surgical repair for perimembranous ventricular septal defect. Ann Thorac Surg 2014;97:1400-6. [Crossref] [PubMed]
- Yang J, Yang L, Yu S, et al. Transcatheter versus surgical closure of perimembranous ventricular septal defects in children: a randomized controlled trial. J Am Coll Cardiol 2014;63:1159-68. [Crossref] [PubMed]
- Narin N, Baykan A, Pamukcu O, et al. ADO II in Percutaneous VSD Closure in Pediatric Patients. J Interv Cardiol 2015;28:479-84. [Crossref] [PubMed]
- Koneti NR, Penumatsa RR, Kanchi V, et al. Retrograde transcatheter closure of ventricular septal defects in children using the Amplatzer Duct Occluder II. Catheter Cardiovasc Interv 2011;77:252-9. [Crossref] [PubMed]
- Polat TB, Turkmen E. Transcatheter closure of ventricular septal defects using the Amplatzer Duct Occluder II device: a single-center experience. Postepy Kardiol Interwencyjnej 2016;12:340-7. [Crossref] [PubMed]
- Ghosh S, Sridhar A, Sivaprakasam M. Complete heart block following transcatheter closure of perimembranous VSD using amplatzer duct occluder II. Catheter Cardiovasc Interv 2017. [Epub ahead of print]. [Crossref] [PubMed]



