Necrotizing pneumonia and empyema caused by Neisseria flavescens infection
Introduction
Neisseria spp. are part of the commensal flora of mucosal membranes of humans and some animals, and are generally considered non-pathogenic except for N. gonorrhoea and N. meningitidis. N. flavescens often be found in the upper respiratory tract and the oropharynx of humans, and are rarely associated with infectious processes (1). However, when patients in special or immunocompromised conditions, N. flavescens can be isolated from blood or cerebrospinal fluid (CSF) occasionally (2-8), but never been isolated from lower respiratory tract.
Herein, we reported a case of a 58-year-old diabetic patient with fatal necrotising pneumonia and empyema due to N. flavescens infection. To our knowledge, this is the first report that N. flavescens as the pathogen of severe low respiratory tract infection.
Case report
A 58-year-old man was admitted to the hospital because of necrotizing pneumonia and empyema in October 2013. He had experienced nausea, vomiting and little cough ten days before admission, after anti-infection therapy with some cephalosporin in local clinic, the symptoms once getting better, but two days before admission, the patient felt anhelation and dyspnea, then presented to the emergency department of our hospital, non symptomatic remission after dealing with cefodizime and methylprednisolone through intravenous injection temporary, then transferred to the department of respiration with symptoms of high grade fever (highest temperature is 39.9 °C/103.82 F), chilling and severe cough with productive of yellow sputum finally.
He has hypertension for four years and controlled well. Four year history of type 2 diabetes and treated with melbine (DMBG) as well as Glipizide, but curative effect is not ideal for fasting blood-glucose more than 10 mmol/L. He also has a smoking history of 20 cigarettes per day for 40 years.
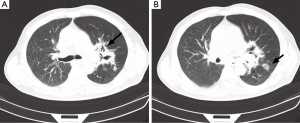
A chest computed tomogram (CT) showed high-density shadow around the hilus of left lung (Figure 1A as signed by black arrow), a hollow sign (Figure 1B as signed by black arrow) also exists in the left peripheral pulmonary. Initial laboratory tests showed the white blood cell (WBC) count was 36.04×109/L (reference level, 4.0×109-10.0×109/L), the neutrophil cell count and ratio was 33.3×109/L (92.4%), the erythrocyte sedimentation rate (ESR) was 115 mm/H, the C-reactive protein (CRP) was 54.1 mg/L (reference level,
A transthoracic pulmonary fine-needle aspiration was performed when transferred to the department of respiration. Approximately 2 mL of purulent secretion was obtained and sent for microbiology tests. Direct smear Gram stain was performed and gram-negative diplococci and lots of polymorphonuclear leukocytes can be observed under microscope (Figure 2A), acid fast stain was also done and got negative results. The same material was inoculated onto chocolate agar and 5% sheep blood agar (bioMérieux, Shanghai, China). The agar media were incubated at 35 °C for 48 h, middle size, bluish grey round opaque colonies were observed. Gram-stain of the pure culture colony was also gram negative cocci. Elementary biochemical properties of this strain were oxidase positive, catalase positive while deoxyribonuclease (DNAse) was negative. The organism was identified with Vitek NH card and Vitek MS successively, but inconsistent results were got, Vitek NH (Ref. V1308 database) identified as N. flavescens (99% probability) while Vitek MS (Ref. V2.0 database) identified as N. subflava (89.70% probability). Finally, we confirmed this identification as N. flavescens (99% probability) by 16S rRNA gene sequencing.
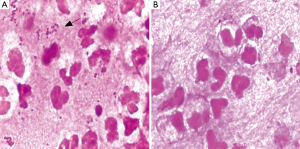
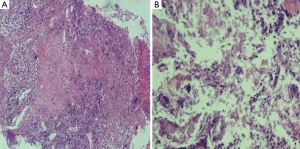
In vitro susceptibility test with agar dilution method was done following the method mentioned in CLSI M45 for Moraxella catarrhalis. It is susceptible to penicillin, ampicillin/sulbactam, amikacin, ceftazidime, ciprofloxacin, Trimethoprim-sulfamethoxazole and piperacillin-tazobatam. After one week anti-infection therapy combined piperacillin-tazobatam and Trimethoprim-sulfamethoxazole, the gram negative diplococcic was almost disappeared (Figure 2B). But the empyema was not released because of the inflamation and necrosis of cartilagines tracheales (Figure 3A,B). Necrosis of cartilagines tracheales lead to tracheal collapse and purulent secretion drainage very uneffective. Finally, the patient was got well after tracheal scaffold implantation and further anti-infective therapy for three weeks.
Discussion
Neisseria is a large genus of commensal bacteria that inhibit mucous membrane surfaces of warm-blooded hosts. There are 11 species that colonize humans include N. gonorrhoeae, N. meningitides, N. lactamica, N. flavescens, N. sicca, N. subflava, N. mucosa, N. cinerea, N. elongata, N. glycolytica and N. nitroreducens. Most of these Neisseria species are normal inhabitants of the upper respiratory tract and are not considered pathogens (1,9). Up to date, only N. meningitides, N. gonorrhoeae, N. mucosa and N. sicca have been reported as causative agents of pneumonia, empyema, bronchopneumonia or bronchiectasis (10-17). Necrotizing pneumonia with empyema caused by N. flavescens is the first time reported as we known. Besides as causative agent of pneumonia and empyema N. flavescens have else been published as pathogens of septicaemia, meningitis and endocarditis (4-8,18-20).
The clinical symptom and lab tests properties of this case are high fever rate, empyema, elevated WBC, increased CRP value and distinctive imaging changes, all these often lead to a fatal infection as reported infection caused by N. flavescens in the other systems (4,6,7,19). We reviewed the literatures and analysed the possible reason may be included the following issues: N. flavescens is among the commensal flora of human upper respiratory tract, seldom cause human infection. Most of N. flavescens infected patients have severe basic diseases, for example, immunodeficiency and diabetes (2); There are remote causes like dental surgery history, vomiting, chemotherapy and co-infection with HIV or pseudomonas aeruginosa (18,21); Initial experienced clinical application of penicillin and cefixime often failed to cure the N. flavescens infection for beta-lactamase producing and penA resistant gene expression (5,22-30); severe virulence and inflammatory response caused by lipooligosaccharide of Neisseria lead to septic shock and fibrinoid necrosis and exudation (31). In conclusion, we should pay more attention to human infection caused by N. flavescens.
Due to N. flavescens may cause severe infection, rapid and accurate identify this organism is more important. As described in this paper, Vitek NH card can be used for accurate identification, but Vitek MS V2.0 database doesn’t include N. flavescens and should be developed in the future. Among the gram negative diplococcus often cause pulmonary infection, N. flavescens can be differentiated from Moraxella catarrhalis with DNAse test, differentiated from N. gonorrhoeae and N. meningitides with rapid acid detection tests and Colistin-susceptible test as summarized in Table 1.

Full table
Acknowledgements
We acknowledge bioMérieux for supplying Vitek MS and related regents. This study was funded by the Key Laboratory for Laboratory Medicine of Jiangsu Province of China (No. XK201114). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure: The authors declare no conflict of interest.
References
- Versalovic J. eds. Manual of Clinical Microbiology Bundle (Print and Digital Edition). ASM Press; 2011.
- Dang AT, Cotton S, Sankaran-Walters S, et al. Evidence of an increased pathogenic footprint in the lingual microbiome of untreated HIV infected patients. BMC Microbiol 2012;12:153. [PubMed]
- Kovalyk AP, Govda AV. Characteristics of microflora of laryngeal mucosa in healthy subjects and patients with cicatrical stenosis of the larynx. Vestn Otorinolaringol 2010;2:17-20. [PubMed]
- Quintero Otero S, Rubio Quiñones F, Hernández Gonzalez A, et al. Septic shock caused by Neisseria flavescens. An Esp Pediatr 1990;33:64-5. [PubMed]
- Sinave CP, Ratzan KR. Infective endocarditis caused by Neisseria flavescens. Am J Med 1987;82:163-4. [PubMed]
- Coovadia YM. Neisseria flavescens septicaemia with meningitis. A case report. S Afr Med J 1984;66:308-9. [PubMed]
- Wertlake PT, Williams TW Jr. Septicaemia caused by Neisseria flavescens. J Clin Pathol 1968;21:437-9. [PubMed]
- Prentice AW. Neisseria flavescens as a cause of meningitis. Lancet 1957;272:613-4. [PubMed]
- Ryan K, Ray CG, Ahmad N, et al. eds. Sherris Medical Microbiology, Fifth Edition. Mcgraw-hill, 2010.
- Gris P, Vincke G, Delmez JP, et al. Neisseria sicca pneumonia and bronchiectasis. Eur Respir J 1989;2:685-7. [PubMed]
- Thorsteinsson SB, Minuth JN, Musher DM. Postpneumonectomy empyema due to Neisseria mucosa. Am J Clin Pathol 1975;64:534-6. [PubMed]
- Enos WF, Beyer JC, Zimmet SM, et al. Unilateral lobar pneumonia with empyema caused by Neisseria gonorrhoeae. South Med J 1980;73:266-7. [PubMed]
- Yagyu Y, Sawaki M, Mikasa K, et al. A clinical study on five cases of respiratory infections caused by Neisseria meningitidis. Kansenshogaku Zasshi 1990;64:822-9. [PubMed]
- Ohtaki M, Tabeta H, Suzuki Y. Bronchopneumonia caused by Neisseria meningitidis--probable transmission by a family member who had been in Hong Kong. Nihon Kyobu Shikkan Gakkai Zasshi 1997;35:461-5. [PubMed]
- Legaria MC, Chadarevian M, Regueira M, et al. Non-pneumonia lower respiratory infection by Neisseria meningitidis. Enferm Infecc Microbiol Clin 1996;14:508-9. [PubMed]
- Ferrer Marcellés A, Andonegui Navarro M, Falcó Ferrer V, et al. Neisseria meningitidis: isolation from low respiratory tract secretions of adult patients. Rev Clin Esp 1996;196:741-6. [PubMed]
- Barnes RV, Dopp AC, Gelberg HJ, et al. Neisseria meningitidis: a cause of nosocomial pneumonia. Am Rev Respir Dis 1975;111:229-31. [PubMed]
- Radke RA, Cunningham GC. A case of meningitis due to Pseudomonas aeruginosa (Bacillus pyocyaneus) and Neisseria flavescens with recovery. J Pediatr 1949;35:99-101. [PubMed]
- Jaroszyńska-Weinberger B, Slubicka A. 2 cases of purulent meningitis caused by Neisseria flavescens in the same environment. Przegl Epidemiol 1968;22:257-9. [PubMed]
- Branham SE, U.S. Public health service. A new meningococcus-like organism (Neisseria flavescens n. sp.) from epidemic meningitis. Washington,: U.S. Govt. print. off.; 1930.
- Szabo S. Lieberman JP, Lue YA. Unusual pathogens in narcotic-associated endocarditis. Rev Infect Dis 1990;12:412-5. [PubMed]
- Spratt BG, Zhang QY, Jones DM, et al. Recruitment of a penicillin-binding protein gene from Neisseria flavescens during the emergence of penicillin resistance in Neisseria meningitidis. Proc Natl Acad Sci U S A 1989;86:8988-92. [PubMed]
- Ameyama S, Onodera S, Takahata M, et al. Mosaic-like structure of penicillin-binding protein 2 Gene (penA) in clinical isolates of Neisseria gonorrhoeae with reduced susceptibility to cefixime. Antimicrob Agents Chemother 2002;46:3744-9. [PubMed]
- Tanaka M, Nakayama H, Huruya K, et al. Analysis of mutations within multiple genes associated with resistance in a clinical isolate of Neisseria gonorrhoeae with reduced ceftriaxone susceptibility that shows a multidrug-resistant phenotype. Int J Antimicrob Agents 2006;27:20-6. [PubMed]
- Spratt BG, Bowler LD, Zhang QY, et al. Role of interspecies transfer of chromosomal genes in the evolution of penicillin resistance in pathogenic and commensal Neisseria species. J Mol Evol 1992;34:115-25. [PubMed]
- Osaka K, Takakura T, Narukawa K, et al. Analysis of amino acid sequences of penicillin-binding protein 2 in clinical isolates of Neisseria gonorrhoeae with reduced susceptibility to cefixime and ceftriaxone. J Infect Chemother 2008;14:195-203. [PubMed]
- Maggs AF, Logan JM, Carter PE, et al. The detection of penicillin insensitivity in Neisseria meningitidis by polymerase chain reaction. J Antimicrob Chemother 1998;42:303-7. [PubMed]
- Lujan R, Zhang QY, Sáez Nieto JA, et al. Penicillin-resistant isolates of Neisseria lactamica produce altered forms of penicillin-binding protein 2 that arose by interspecies horizontal gene transfer. Antimicrob Agents Chemother 1991;35:300-4. [PubMed]
- Bowler LD, Zhang QY, Riou JY, et al. Interspecies recombination between the penA genes of Neisseria meningitidis and commensal Neisseria species during the emergence of penicillin resistance in N. meningitidis: natural events and laboratory simulation. J Bacteriol 1994;176:333-7. [PubMed]
- Genco CA, Knapp JS, Clark VL. Conjugation of plasmids of Neisseria gonorrhoeae to other Neisseria species: potential reservoirs for the beta-lactamase plasmid. J Infect Dis 1984;150:397-401. [PubMed]
- Zarantonelli ML, Huerre M, Taha MK, et al. Differential role of lipooligosaccharide of Neisseria meningitidis in virulence and inflammatory response during respiratory infection in mice. Infect Immun 2006;74:5506-12. [PubMed]


