Echocardiography guidance of atrial septal defect closure
Introduction
Continual growth of cardiac interventions, have paralleled advancements in cardiac imaging. Transcatheter procedures offer less invasive approaches allowing rapid recovery and earlier hospital discharge. A number of international guidelines (1-3) have defined the role of echocardiography spanning diagnosis, detailed anatomical assessment, device sizing and selection, peri-procedure guidance and post device surveillance. Its appropriate use has been shown to improve efficiency and safety through reduction in procedure times, radiation exposure and improved early and longer-term outcomes (4). While two-dimensional echocardiography (2D Echo) and fluoroscopy imaging remain essential tools during structural heart interventions, increasingly three-dimensional echocardiography (3D echo) plays a central role. Although intra-cardiac echocardiography (ICE) may offer some potential peri-procedure advantages; however its use remains limited (Table 1) (5). Indeed transoesophageal echocardiography (TEE) imaging provides unrivalled multiplane, high-resolution imaging and will be the focus of this article.

Full table
Common to all forms of interventional imaging is a precise understanding of
- The morphological phenotypes relating to the structure of interest;
- The key anatomical landmarks that aid spatial orientation, supported by a comprehensive systematic echo protocol;
- Device sizing based on imaging measurements;
- Specific imaging views required during procedure guidance.
There is no doubt the present era demands delivery of high quality interventional echocardiography to enable continued advancements in this field. This review aims to provide the core knowledge necessary in delivering optimal procedural echo imaging in supporting best clinical outcomes for transcatheter atrial septal defect (ASD) closure.
Morphological phenotypes of atrial septum (AS) defects
The presence of an ASD, whatever the type, is typically suggested by the finding of a dilated and volume overloaded right ventricle during transthoracic echo (TTE) study (see Figure 1A,B,C). The differential diagnosis may include tricuspid and pulmonary regurgitation or the presence of a shunt either anomalous pulmonary venous drainage or ASD. The ASD may be visualized in three main echo views (see Figure 1D,E,F). As part of the assessment other essential findings should be documented, including; concomitant mitral valve (MV) disease (significant disease necessitating surgical repair may be hampered by the presence of a large ASD device) where a surgical approach to repairing both lesions may be more appropriate; dilated left atrium and left ventricle with evidence of diastolic dysfunction; or right ventricular function impairment and evidence of significant pulmonary hypertension; evidence of other congenital lesions [such as bicuspid aortic valve (AV) disease, associated aortopathy, patent ductus arteriosus, ventricular septal defect]. Such findings may alter management in terms of appropriateness of ASD closure or a preferred surgical approach.
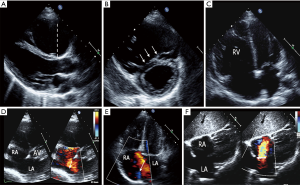
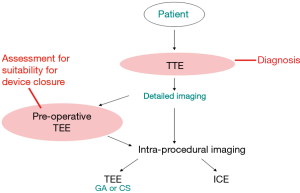
Although TTE is often diagnostic, see Figure 1 (images A-F), TEE is necessary to confirm the anatomy and assess suitability for device closure in most adults, as image resolution is limited. Figure 2 gives an overview for the role of the echo modalities in workup of such patients. Four major types of ASD are described and should be distinguished (6), see Figure 3 (images A, C, E, I). A prerequisite for transcatheter device closure is the presence of adequate rims of tissue bordering the defect. Thus, only secundum ASD (sASD) may be suitable for percutaneous device closure and differ considerably in size, shape, location and number, see Figure 3 (images C, D, G, I).

The European Society of Cardiology (ESC) Class I indications (7) for secundum atrial septal defects (sASD) closure include presence of a significant shunt (signs of RV volume overload) and when pulmonary vascular resistance is less than 5 Wood Units, where the method of choice is transcatheter device closure whenever possible. See Table 2 for summary of indications.

Full table
Key anatomical landmarks and TEE protocol
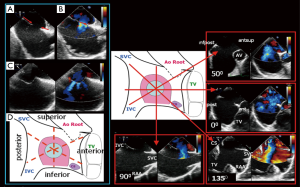
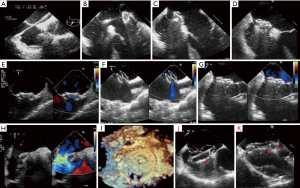
Key landmarks are defined by their close proximity to the atrial septum (AS). On the right atrial (RA) side the superior vena cava (SVC), inferior vena cava (IVC), aortic root (Ao) and AV, coronary sinus and tricuspid valve are relevant. While on the left atrial (LA) side the right upper pulmonary vein (RUPV) and MV are important structures (Figure 4). A systematic approach to imaging the AS is encouraged (8) to ensure a complete assessment avoids the risk of missing complex anatomy (Figure 4). The protocol includes a scout at 0 degrees in transverse plane, imaging the AS through its entire length (high, mid, low esophageal levels allowing a quick overview of the defect) then rotating through 0°–180° preferably in 10°–15° increments where specific rim assessments are viewed at 45°, 90°, 135°. A number of anatomical findings should be recorded as each may impact on device type and size selected. A checklist is provided in Table 3. Specific rims of the sASD are measured on 2D imaging, and are defined as a minimum of 5 mm in length (Figure 4A,B,C,D). This ensures adequate surrounding septal tissue for a device to anchor. Consideration should be given to the integrity of the rim tissue. Thin flimsy tissue is unlikely to have the strength to hold the device disc despite appearing an adequate length. Traditional teaching allows the aortic rim to be absent since the ASD closure device is able to anchor using both left and right discs by splaying across the aortic root. However, more recently a number of erosions resulting in cardiac perforations have led to caution in such anatomy (9,10). The incidence is low 0.1–0.3% (11), and usually arises within the first 12 months although has been reported as late as 8 years post procedure. High risk features appear to include absent or limited aortic and superior vena cava rims (12), compounded by significant device oversizing, and motion (‘see-saw’ movement) where the waist of the device (or the disc edges protrude into the aortic wall) is in continual contact with the aortic root, perhaps causing distortion of its wall (9,10) (Figure 5). Several discrete defects may necessitate multiple device implantations or fenestrations (multiple small ‘pepper pot’ defects) may warrant a large device with a narrow waist placed as centrally as possible on the AS to ensure coverage of the entire fossa ovalis (FO).

Full table
Device sizing
Sizing the sASD varies between institutions and is dependent on availability and quality of echocardiography imaging offered and preferences of the interventional cardiologist during the procedure. Two major approaches exit, TEE sizing or balloon sizing (BS). 2D TEE provides high-resolution images where the defect measures slightly smaller than the actual size required for device sizing. Colour flow Doppler (CFD) clearly demonstrates flow and the boundaries of the ASD, being comparable to surgical (13) and device sized measurements (14). When the measurements in all four TEE planes [0, 45, 90, 135] are similar (1–2 mm) the largest is taken as the ASD size. If the measurements are significantly different (≥3 mm) a mental reconstruction will allow an understanding of the overall 3D shape, although 3D TEE has superseded this requirement, see below. When the defect is circular a single diameter measurement is taken. When oval in shape the short and long axes are averaged and correlate with device sizing. The device size chosen is the measured ASD size plus 20% (15,16). If additional features exist [such as atrial septal aneurysm (ASA) or absent aortic rim] we usually add 25% to the measured size of the defect thereby allowing for adequate grip of the surrounding rims.
Alternatively BS at the time of the procedure is an option. However unless the defect opens (folds back) noticeably once the guide wire is placed, reliance can be placed on echo measurements without the need for BS. If BS is employed then this should be a ‘stop-flow’ technique (17). This approach ensures sizing in line with TEE avoiding oversizing and its subsequent risks (18). When performed correctly the method relies on TEE imaging. The shaft of the balloon, as it is inflated, is visualized through its entirety (confirming alignment through the central long axis for accurate sizing) and monitored using CFD. When the flow on CFD stops balloon inflation ceases (Figure 5K). This method prevents oversizing and a prominent balloon waist is avoided (suggesting over-inflation). The narrowest central portion of the balloon is measured on TEE and compared to fluoroscopy. The measured diameter equates to the device size. It is recommended not to oversize more than 2–4 mm in the case of deficient anterosuperior rim due to an increased risk of erosion.
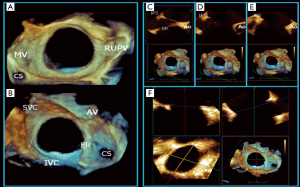
A 3D anatomical understanding of the defect is indispensable when deciding which measurements to include (19). 3D echo provides instantaneous appreciation of defect position, orientation, size and spatial relationships to surrounding structures (20,21) (Figure 6), and may offer a superior approach to ASD measurements. Correct anatomical orientation of the 3D image is essential and ideally performed and presented for viewing in a standardised manner. We adopt a similar approach to all 3D echo imaging and like the ‘surgical view’ of the MV, the AS is presented in an anatomically correct way with clearly defined landmarks (en face LA and RA views, Figure 6A,B). Hence a major advantage of 3D echo, is its ability to simultaneously align anatomical understanding between team members (interventional, surgical and imaging) enabling more effective dialogue and an efficiently performed procedure. 3D multi-plane reconstruction allows a detailed assessment of the ASD rims (Figure 6C,D,E), simplifying the 2D incremental 10°–15° sweep performed to assess the precise nature of each rim region. Accurate orientation of the 3D defect in its true long and short axis can be rapidly achieved (Figure 6F). 3D TEE ASD measurements appear to be more accurate when compared to 2D since they allow precise orientation with the defect axes (22-24). Latest 3D software allows immediate direct measurements to be made from the live 3D image and may make this approach more accessible during procedures.
Procedure guidance
Echo guidance during the procedure optimizes visualisation of the guide wire (and delivery sheath) from the IVC across the defect and into the LA, positioned in the left upper pulmonary vein. Typically this is approximately TEE 45° view. By keeping the delivery sheath in view the device is unsheathed (usually seen towards LA roof) and LA disc is deployed, guided back to and once in apposition to the AS, the RA disc is deployed (Figure 5A,B,C,D). A systematic check using the protocol described assesses the device form to ensure it has reshaped to the correct profile, its position on the AS, relationship to surrounding structures and presence of residual flow and its possible cause. CFD reveals flow through the device and is expected (Figure 5E). However flow seen around the free edges of the device maybe abnormal and should be assessed systematically to ensure the device has been correctly sized, and no other defects have been unmasked by alterations in AS geometry (Figure 5F,G,H,I). 3D TEE gives excellent overview of all these aspects complimenting the information acquired on 2D TEE. Once satisfied the device position and size are correct the device is released and final TEE imaging checks are made repeating the above protocol. Prior to discharge TTE confirms correct device position and excludes pericardial effusion.
Limitations of TEE imaging should be remembered and include poor echo windows; especially low esophageal views where the inferior border of the AS moves away from the esophageal wall and may be difficult to image. LA size effects the angle of view and ensuring the patient is appropriately volume loaded is essential to optimize the widest angle of view as possible. Where image quality remains poor or esophageal intubation is contra-indicated or where the TEE probe cannot be tolerated (e.g., general anaesthesia not advisable) then ICE imaging may be considered. However referral to a high volume centre with expertise in the use of ICE for ASD closure is advisable if experience is limited.
Post-procedure follow up
In our institution follow up is performed at 6 weeks where both a clinical assessment and TTE are performed. If all appears satisfactory (i.e., device position is satisfactory with no residual flow), then a further TTE is performed at six months. Typically the right ventricle has reduced in size to normal or near normal. Tricuspid regurgitation, usually mild or moderate on pre-closure baseline TTE, has improved and is associated with RV and TV annular size reduction. Pulmonary hypertension is less often seen and if noted requires more frequent follow up. If RV has returned to normal size, the device positioned correctly with no or mild TR only then usual follow up is between 2–3 years. At this stage if no issues are found then the patient may be discharged to local follow up. In the case of large devices, where there is concern of potential erosion the patient is counselled regarding the development of concerning symptoms including chest pain, collapse or palpitations and will be followed up annually.
Conclusions
Transcatheter structural heart interventions afford the benefits of minimally invasive procedures and in the case of the ASD closure is the method of choice. Echocardiography plays a central role in optimizing procedure safety and success through reduced radiation exposure, procedure times and complication rates along with accurate device selection. The addition of 3D TEE imaging has further enhanced our understanding of this procedure. When approached in the manner described above: defining morphological phenotypes, anatomical landmarks, systematic TEE protocol with precise anatomical description and defect sizing, it serves to deliver optimal procedural echo imaging.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has received honoraria for lecturing and providing teaching material to Occlutech Company, Boston Scientific Company Inc. and St. Jude Medical (now Abbott Vascular) Company.
References
- Zamorano JL, Badano LP, Bruce C, et al. EAE/ASE recommendations for the use of echocardiography in new transcatheter interventions for valvular heart disease. Eur Heart J 2011;32:2189-214. [Crossref] [PubMed]
- Lang RM, Badano LP, Tsang W, et al. EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. Eur Heart J Cardiovasc Imaging 2012;13:1-46. [Crossref] [PubMed]
- Silvestry FE, Cohen MS, Armsby LB, et al. Guidelines for the Echocardiographic Assessment of Atrial Septal Defect and Patent Foramen Ovale: From the American Society of Echocardiography and Society for Cardiac Angiography and Interventions. J Am Soc Echocardiogr 2015;28:910-58. [Crossref] [PubMed]
- Biner S, Perk G, Kar S, et al. Utility of combined two-dimensional and three-dimensional transesophageal imaging for catheter-based mitral valve clip repair of mitral regurgitation. J Am Soc Echocardiogr 2011;24:611-7. [Crossref] [PubMed]
- Bartel T, Müller S, Biviano A, et al. Why is intracardiac echocardiography helpful? Benefits, costs, and how to learn. Eur Heart J 2014;35:69-76. [Crossref] [PubMed]
- Geva T, Martins JD, Wald RM. Atrial septal defects. Lancet 2014;383:1921-32. [Crossref] [PubMed]
- Baumgartner H, Bonhoeffer P, De Groot NMS, et al. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J 2010;31:2915-57. [Crossref] [PubMed]
- Rana BS, Thomas MR, Calvert PA, et al. Echocardiographic evaluation of patent foramen ovale prior to device closure. JACC Cardiovasc Imaging 2010;3:749-60. [Crossref] [PubMed]
- Moore J, Hegde S, El-Said H, et al. Transcatheter device closure of atrial septal defects: A safety review. JACC Cardiovasc Interv 2013;6:433-42. [Crossref] [PubMed]
- Diab K, Kenny D, Hijazi ZM. Erosions, erosions, and erosions! Device closure of atrial septal defects: how safe is safe? Catheter Cardiovasc Interv 2012;80:168-74. [Crossref] [PubMed]
- Turi ZG, Peters P. Look before (and after) you plug: Moving slowly toward safer selection and management of patients at risk of device erosion. Catheter Cardiovasc Interv 2014;83:93-4. [Crossref] [PubMed]
- Divekar A, Gaamangwe T, Shaikh N, et al. Cardiac perforation after device closure of atrial septal defects with the Amplatzer septal occluder. J Am Coll Cardiol 2005;45:1213-8. [Crossref] [PubMed]
- Faletra F, Scarpini S, Moreo A, et al. Color Doppler echocardiographic assessment of atrial septal defect size: correlation with surgical measurements. J Am Soc Echocardiogr 1991;4:429-34. [Crossref] [PubMed]
- Tzifa A, Gordon J, Tibby SM, et al. Transcatheter atrial septal defect closure guided by colour flow Doppler. Int J Cardiol 2011;149:299-303. [Crossref] [PubMed]
- Quek SC, Wu WX, Chan KY, et al. Transcatheter closure of atrial septal defects--is balloon sizing still necessary? Ann Acad Med Singapore 2010;39:390-3. [PubMed]
- Hajizeinali A, Sadeghian H, Rezvanfard M, et al. A comparison between size of the occluder device and two-dimensional transoesophageal echocardiographic sizing of the ostium secundum atrial septal defect. Cardiovasc J Afr 2013;24:161-4. [Crossref] [PubMed]
- Carlson KM, Justino H, O’Brien RE, et al. Transcatheter atrial septal defect closure: Modified balloon sizing technique to avoid overstretching the defect and oversizing the amplatzer septal occluder. Catheter Cardiovasc Interv. 2005;66:390-6. [Crossref] [PubMed]
- Wang JK, Tsai SK, Lin SM, et al. Transcatheter closure of atrial septal defect without balloon sizing. Catheter Cardiovasc Interv 2008;71:214-21. [Crossref] [PubMed]
- Rana BS. Three-dimensional echocardiography and structural heart interventions. Br J Hosp Med (Lond) 2014;75:378-83. [Crossref] [PubMed]
- Roberson DA, Cui W, Patel D, et al. Three-dimensional transesophageal echocardiography of atrial septal defect: a qualitative and quantitative anatomic study. J Am Soc Echocardiogr 2011;24:600-10. [Crossref] [PubMed]
- Roberson DA, Cui VW. Three-dimensional transesophageal echocardiography of atrial septal defect device closure. Curr Cardiol Rep 2014;16:453. [Crossref] [PubMed]
- Zhu W, Cao QL, Rhodes J, et al. Measurement of atrial septal defect size: a comparative study between three-dimensional transesophageal echocardiography and the standard balloon sizing methods. Pediatr Cardiol 2000;21:465-9. [Crossref] [PubMed]
- Cao Q, Radtke W, Berger F, et al. Transcatheter closure of multiple atrial septal defects. Initial results and value of two- and three-dimensional transoesophageal echocardiography. Eur Heart J 2000;21:941-7. [Crossref] [PubMed]
- Saric M, Perk G, Purgess JR, et al. Imaging atrial septal defects by real-time three-dimensional transesophageal echocardiography: step-by-step approach. J Am Soc Echocardiogr 2010;23:1128-35. [Crossref] [PubMed]


