The utilization of resuscitative endovascular balloon occlusion of the aorta: preparation, technique, and the implementation of a novel approach to stabilizing hemorrhage
Introduction
With advances in technology and medical science, treatment methods of trauma patients are constantly improving; however, noncompressible torso hemorrhage (NCTH), such as a massive hemorrhage associated with pelvic ring, abdominal, and thoracic injuries, remains the main cause of preventable death (1-4). Therefore, rapid and effective procedures are needed to achieve hemostasis in cases of traumatic torso hemorrhage. Resuscitative thoracotomy (RT) followed by aortic cross clamping (ACC, hereafter referred to as RT-ACC) might be performed as damage control surgery (DCS) to decrease the hemorrhage below the clamping site and to increase cerebral and coronary perfusion in these patients (5,6). Unfortunately, RT still demonstrates high morbidity and mortality rates (5,7); it is especially limited in patients with hemorrhagic shock and cardiac arrest from trauma (7). Presently, resuscitative endovascular balloon occlusion of the aorta (REBOA) has been gaining acceptance as an alternative to ACC to achieve hemostasis in NCTH patients presenting in extremis (3-6,8-11). It offers a less invasive approach for salvaging severely injured patients with hemorrhage compared with RT (4,5,12). REBOA could be more effective and easier to perform than ACC as an aspect of occlusion level for bleeding control and total/partial/intermittent occlusion to avoid organ ischemia (5,13,14). Furthermore, field REBOA/prehospital REBOA may be an option to manage torso hemorrhage (15,16). Despite its effectiveness in end-stage hemorrhagic shock, the application of REBOA in trauma patients remains limited. The aim of study was to provide a conceptual understanding, environmental settings and technical methods in implementing REBOA.
Evolution of REBOA
Intra-aortic balloon occlusion (IABO) in patients with hemorrhagic shock was reported as early as 1954 during the Korean War (17). Subsequently, IABO was used preoperatively to control hemorrhage from a ruptured abdominal aortic aneurysm (AAA), intraoperatively for AAA repair, and for postpartum hemorrhage (18-20). Further, the recent evolution of endovascular technology has enabled the development of REBOA in the emergency department to gain time for resuscitation. During the past 5 years, numerous clinical studies on REBOA in the trauma setting support its advantage in restoring hemodynamics (4,6,9-11,14,21-24). In a patient with hemorrhagic shock from the abdomen and/or pelvis, DCS such as crash laparotomy and pelvic fixation followed by pre-peritoneal pelvic packing is ideal. However, DCS cannot always be performed immediately because of limitation of the operative environment or patient’s condition, such as those with a difficult airway or a post-intubation hypotension. Therefore, REBOA was proposed as a possible bridging therapy that may support the patient’s hemodynamics (11,25).
Effect of REBOA
Aortic occlusion with an intravascular balloon provides several beneficial effects. First, the procedure raises central arterial pressure and improves cerebral and myocardial perfusion. Second, active hemorrhage below the inflated balloon is decreased. Third, REBOA is less invasive than RT-ACC. Fourth, aortic occlusion is permitted at different levels depending on hemorrhage focus.
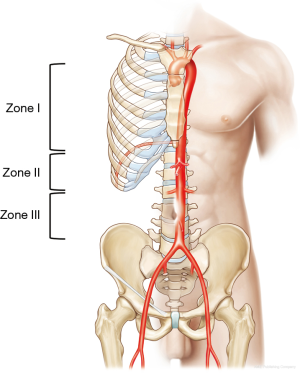
REBOA effectively blocks blood flow to the lower body at the level of the three aortic zones (11): zone I, the descending thoracic aorta, from the origin of the left subclavian artery to the top part of the celiac artery; zone II, the abdominal aorta, extending from the celiac artery to the lowest renal artery; and zone III, the infrarenal abdominal aorta (Figure 1). Zone I REBOA is effective for controlling abdominopelvic hemorrhage, whereas zone III REBOA is useful for controlling pelvic hemorrhage in extremis. Occlusion in zone II should be avoided because of potential organ ischemia.
REBOA algorithm
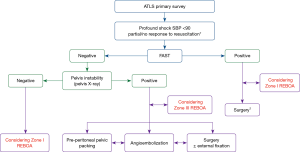
There are several REBOA algorithms to manage hemodynamic instability (12,13,26). In general, REBOA may be limited in patients with cardiac/aortic or thoracic injury, but could be useful in patients with abdominopelvic hemorrhage (27). REBOA is recommended after initiating a massive transfusion for the management of hemodynamically unstable patients with unstable pelvic fracture and torso hemorrhage, according to the Denver Health Medical Center algorithm (26). The University of Maryland Shock Trauma algorithm for REBOA shows that REBOA is contraindicated in cases with possible aortic injury on a chest radiograph and that zone I REBOA is indicated in patients with hemoperitoneum on focused assessment with sonography for trauma (FAST) or a hemodynamically unstable pelvic bone fracture (13). At our institution, the Advanced Trauma Life Support (ATLS) survey should be performed first in patients with severe hemorrhagic shock. If no cardiac tamponade or thoracic aortic injury is observed on a trauma series (chest radiograph) or FAST, and the patient has no or partial response to resuscitation despite early transfusion with three units of O-positive packed red blood cells and three units of AB-positive fresh frozen plasma in the resuscitation room, REBOA could be subsequently considered to save the patient (Figure 2). Zone I REBOA in a patient with no or a partial response to hemoperitoneum, and Zone III REBOA in a patient with persistent shock due to uncontrolled pelvic hemorrhage may be considered. According to one report (12), even when the patient shows no hemoperitoneum on FAST or no pelvis instability on radiography, zone I REBOA might be considered because increasing the arterial pressure enables evaluation of the cause of shock, such as retroperitoneal hemorrhage, with radiological imaging.
Resuscitative time is important to improve the survival rate of hemodynamically unstable trauma patients during initial evaluation and treatment. Saito et al. (28) reported that the median time from the door to balloon inflation was approximately 20 min, and the time to reach the decision to use REBOA is most essential. In addition, door-to-surgery time is important; if this time is 60 min or greater, there is a high in-hospital mortality rate due to delay in hemorrhage control (22). At our institution, if the door-to-incision time is expected to be less than 30 min, surgery could be a first choice. However, if the time is expected to be delayed more than 30 min, zone I REBOA may be considered. Since REBOA is a bridging therapy for damage control resuscitation, hemorrhage control management, such as surgery or embolization, should be performed as soon as possible.
Preparation
In general, imaging equipment, contrast media, and a REBOA kit are necessary to perform REBOA in ideal conditions. The imaging equipment and technique needed in the sequence of the procedure consists of ultrasound, C-arm, angiography, and serial X-ray. The REBOA kit includes a sheath, wire, balloon, and cutdown pack.
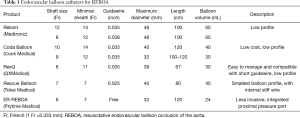
The REBOA kit is readily available in the emergency department and operating room. If the patient is hemodynamically unstable, vascular access with REBOA is considered first. In general, a simple drape is needed for arterial puncture and sheath insertion. Sheaths are sized based on their internal diameter as French (Fr; 1 Fr =0.333 mm). There are variable sheaths, guidewires, and balloons (Table 1). When selecting sheath size, the decision depends on the balloon size. If a 12-Fr balloon is available, a 14-Fr sheath and 0.035-inch wire should be ready. For a 7-Fr small balloon catheter, a 7- to 9-Fr sheath and a 0.025-inch wire are needed. This concept is important when selecting the balloon. To occlude blood flow, the intra-aortic balloon must be soft and compliant, and must have a large diameter (11,13). A balloon catheter that is too small cannot occlude blood flow, and a stiff or noncompliant balloon may cause damage to the aorta, such as dissection and rupture. Presently, small and compliant balloon catheter with a large diameter (32–40 mm)—the 7-Fr RESCUE balloon (Tokai Medical Products, Aichi, Japan) and the ER-REBOA catheter (Prytime Medical Devices, Boeme, TX, USA)—were developed. Especially, the ER-REBOA catheter is a wireless system that eliminates the need to place the wire. Therefore, this device may be useful for field REBOA, such as in military medicine.
Full table
To complete REBOA, a routine drape for covering the lower body below the umbilicus is needed. Since the wire and balloon catheter are long, full draping below the umbilicus is recommended. If the Seldinger technique to advance into the common femoral artery (CFA) fails, when possibly accessing it on only one side, switching to an open technique for vascular access is necessary. Therefore, a surgical gown and vascular cutdown pack, including Metzenbaum scissors and a mastoid retractor, are needed.
Guidewire positioning must be checked with imaging equipment, such as C-arm, angiography, sonography, and X-ray, not accessing the contralateral common iliac artery or venous system. Even though real-time fluoroscopic guidance using C-arm or angiography or hybrid operating rooms are ideal requirements, these are usually not available in the resuscitation room. Ogura et al. (29) reported that ultrasound-guided REBOA could determine the placement of the balloon catheter without fluoroscopy. However, sonographic windows may be poor in patients with pneumoperitoneum or subcutaneous emphysema, obesity, or fatty liver. In this situation, serial X-ray is a practical option to confirm positioning. Then, a selected balloon catheter is inserted along the guidewire and inflated. Inflation with only saline enables positioning of the balloon catheter with radiopaque markers. If available during inflation, iodinated contrast is more useful to check balloon positioning. The contrast would be mixed with saline at a ratio of 1:1 (13).
Procedure
Arterial access and sheath positioning
The first step involves identification of the vessel and assessment of peripheral circulation. Arterial access is preferable to the CFA on the contralateral side of the injury site. If the injury involves bilateral inguinal areas, REBOA with axillary or brachial access may be an option. In patients with hemorrhagic shock, palpation of the arterial pulse is difficult. To avoid vascular injury and establish vascular access, ultrasound-guided percutaneous arterial puncture is recommended as the most effective modality to access the vessel in an emergency (29,30). If ultrasound is not available, the inguinal ligament is distinguished by palpation and a puncture attempt may be challenging. The puncture site is recommended to be approximately 2 fingerbreadths or 2 to 3 cm below the ligament due to the location of the CFA (11,13). The CFA is relatively easy to access in patients with hemorrhagic shock. Furthermore, the superficial femoral artery may be associated with a greater risk of leg ischemia because its diameter is smaller than that of the CFA in cases of shock. If access to the CFA is achieved, the sheath is subsequently advanced through it and secured with a suture.
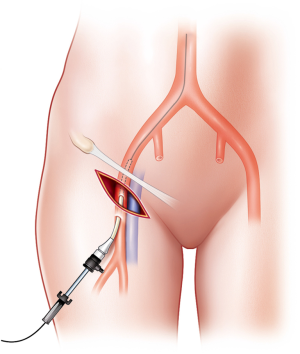
However, the Seldinger technique may be difficult and may fail in patients with profound shock and obesity. First, open exposure of the vessel should be immediately performed without hesitation. An early cutdown approach might be a good choice to save operative time by avoiding time-consuming and repeated arterial punctures. If a skin incision is made transversely below the inguinal ligament, the CFA is identified by dissecting onto the artery. Thereafter, puncture via the Seldinger technique and sheath insertion may be completed (Figure 3). Second, if the sheath is located in the femoral vein, it may be not be necessary to remove it as it could be useful in fluid administration and in identification of the vascular anatomy. Vascular access for sheath insertion is time-consuming (4,31). Matsumoto et al. (31) reported the “Prompt and Rapid Endovascular Strategies in Traumatic Occasions” (PRESTO) concepts as a time-conscious algorithm. Even if the patient does not arrive at the hospital, earlier activation of an interventional radiology team could be beneficial for rapid completion of hemostasis because of more rapid diagnosis and intervention. Furthermore, cannulation of the CFA for diagnostic and therapeutic capabilities is useful for monitoring arterial blood pressure and for performing treatment methods, such as embolization and REBOA. Therefore, a small sheath, such as 5-Fr sheath, is usually favorable at first. If a larger size is needed for aortic occlusion, the sheath can be upsized. If the patient has an existing arterial line on the CFA, it can be used for REBOA sheath insertion, because the upgrading time may be shorter than that for initial vascular access with a large bore sheath in patients with shock.
Guidewire and balloon positioning
In this process, there are two important cautions: one is resistance and the other is that the balloon should not be inflated for testing. A guidewire usually is usually required to place a balloon, except for the ER-REBOA catheter. The guidewire is slowly advanced through the sheath. Real-time fluoroscopic guidance using C-arm or angiography is more useful for tracing the aortic pathway of the guidewire or balloon catheter and can be used to confirm their position. If real-time imaging equipment is not available in the emergency setting, serial X-ray or ultrasound is substantially useful (32). If advancement of the guidewire is met with resistance, further advancement should be stopped because the wire is not located in the vessel or it may damage the vessel wall even while in the vessel. After guidewire placement in the aorta is confirmed, a balloon catheter is inserted along the guidewire and the balloon is positioned at the aortic zone of the block. At this time, a balloon test must not be performed using a 7-Fr RESCUE balloon catheter because an inflation-tested balloon catheter cannot enter through the 7-Fr sheath. To solve the problem, when the balloon has been inflated for testing, the balloon should be replaced with a new one or a larger sized sheath should be used. After insertion of the balloon catheter along the guidewire, with a small catheter, such as the 7-Fr RESCUE balloon, the guidewire in the balloon catheter should be changed to a stiff wire to prevent caudal migration of the balloon catheter.
Accurate balloon positioning is important to minimize perfusion-related complications, such as ischemia. For example, balloon positioning is recommended for placement above diaphragm level in zone I. If imaging equipment is not available immediately, simulation with the anatomic marker of the balloon catheter can be used to estimate balloon positioning. The proximal marker of the balloon catheter for zone I level should be above the xyphoid process and that for zone III is the umbilicus; therefore, it may be useful to measure the estimated length prior to insertion. Pezy et al. (33) reported that the length of the segment from the point of entry of the catheter to the balloon position is 414 to 474 mm in zone I and 236 to 256 mm in zone III. Therefore, a marking on the balloon catheter following simulation may indicate the actual length of a positioned catheter.
Balloon inflation
The maximal volume for inflation depends on the balloon catheter and aortic diameter in the patient. Since aortic diameter may be decreased in cases of hemorrhagic shock, a small volume to inflate the balloon may be necessary. In general, the balloon ideally should be inflated within the maximal volume of the balloon catheter until hemorrhage control is achieved. If inflated resistance of the syringe is felt, balloon inflation should be stopped and the situation checked with fluoroscopy or X-ray. Even if a balloon catheter is soft and compliant, the ballooning pressure may cause aortic injury. In addition, organ ischemia below the occlusion is inevitable, and prolonged aortic occlusion may lead to ischemic change in nonperfused tissues. Therefore, intermittent or partial inflation/deflation of the balloon can be performed to provide organ perfusion. According to several reports (21,28), the proper mean aortic occlusion time is suggested to be 18 to 21 min, whereas Qasim et al. (34) reported no significant procedure-related complications if the aortic occlusion time was not over 40 min. Presently, partial (pREBOA) and intermittent (iREBOA) REBOA concepts can be regarded to decrease ischemic events (14,35). This is the same concept with permissive hypotensive treatment; systolic blood pressure is not less than 80 mmHg to accomplish balloon inflation. However, accurate and appropriate volume and time for pREBOA and iREBOA have not yet been established. After completion of balloon inflation, to secure the sheath and balloon, a suture and occlusive dressing must be placed. This securing field must be closely observed because the increasing blood pressure may cause caudal migration of the balloon catheter.
Balloon deflation
If the patient’s hemodynamics is stable, balloon deflation may be considered. During this process, it is important and critical that the REBOA operator communicate with the trauma surgeon, anesthesiologist, and interventional radiologist. Deflation may result in a sudden decrease in blood pressure when damage control resuscitation has not yet been completed. Abrupt deflation may result in significant hypotension. Therefore, the balloon should be deflated slowly and cautiously. At our institution, the balloon is deflated slowly at 1 to 2 mL/2 to 3 min with intensive monitoring of the proximal arterial blood pressure. Furthermore, from vascular access to deflation, the operator as the balloon holder should be designated to control all steps, including communicating with the trauma surgeon, anesthesiologist, and interventional radiologist during surgery or any intervention.
Balloon catheter and sheath removal
The balloon catheter can be removed when the patient is hemodynamically stable and REBOA is no longer required. The sheath can be removed when additional intervention is no longer required and the patient’s coagulation profile is normal. If the patient’s hemodynamics does not stabilize before catheter removal, intermittent balloon inflation and deflation may be necessary until hemodynamics stabilizes. The sheath, especially a large sheath, should be removed to avoid leg ischemia and thrombus complications as soon as possible. Before sheath removal, 100 mL of heparinized saline (1,000 units heparin in 1 L saline) should be flushed to prevent thrombus formation (11).
There are several methods of sheath removal, such as external compression, direct suture repair, and use of devices. External compression is appropriate in case of a small sheath, but direct suture repair is more secure in case of a large sheath or the cutdown technique. Presently, there are several practical methods using closure devices, such as the Perclose ProGlide (Abbott Park, Chicago, IL, USA) and StarClose SE (Abbott Park). After sheath removal, peripheral circulation should be monitored by manual palpation for pulse and Doppler ultrasound.
Outcome
Although there have been a few observational studies on REBOA, clinical cohort studies are lacking owing to its short history (Table 2).

Full table
In general, many investigators have compared RT-ACC with REBOA (6,8-10). Some studies (6,10) have reported that REBOA improves survival rate compared with RT-ACC. On the other hand, Norii et al. (23) reported that mortality rate is higher in patients who have undergone REBOA than in similarly injured patients who have not undergone REBOA. Furthermore, there was a vast variation in the overall survival rates (13–85.7%) according to characteristics of enrolled populations, duration of aortic occlusion time, or arranged zone (14,36,37). There are several reasons for the different findings of those studies. First, characteristics of trauma patients indicate heterogeneous populations. There are many demographic differences, such as age and mechanism of injury. Second, to our knowledge, there is no randomized controlled trial study. Most studies are small-sized, retrospective, or multicenter registries with different protocols, except for the prospective study by the American Association for the Surgery of Trauma (AAST) Aortic Occlusion for Resuscitation in Trauma and Acute Care Surgery (AORTA) Registry (9). In those studies, overall survival rate was not significantly different between REBOA and ACC, but there were also limitations, such as differences in modality, and endpoint of outcomes, such as survival and/or neurologic function. Third, there were different medical environments. For example, arrival-to-surgery time at each center was different because of possible delay due to few in-house trauma surgeons, and subjects who performed primary aortic occlusion were different among residents, training fellows, or Trauma/Vascular surgery attending physicians. However, the fact is that REBOA is less invasive than ACC and could be used in patients with unstable pelvic fractures or abdominal injury as a viable alternative to ACC. Therefore, to promptly treat moribund trauma patients with massive hemorrhage, the trauma team—including interventional radiologists, emergency physicians, and trauma surgeons—should be organized so that they can make timely decisions regarding the multidisciplinary approach and procedure of endovascular management, such as REBOA, to ultimately decrease the resuscitative time (31,42).
Complication
Complications following REBOA are divided into two groups: procedure-related and perfusion-related complications. Several studies (28,29,36,38) have reported on procedure-related complications. The most common cause is failed arterial access (40%), and other causes are vascular injuries during arterial access and sheath positioning. Since arterial access in patients with profound shock is usually challenging, an ultrasound-guided technique should be recommended to reduce procedure-related complications (29). Teeter et al. (39) reported that a smaller sheath, such as a 7-Fr sheath, for REBOA is appropriate for aortic occlusion and may be associated with fewer access-related complications despite the longer duration of sheath placement (mean, 25 h; range, 17–41 h). Therefore, small sheaths with relevant balloon catheters may be safe and effective for REBOA.
Perfusion-related complications, including acute kidney injury (25%), leg ischemia with embolism (8%), multiple organ failure (38%) in the occlusion phase, and reperfusion, have been reported (9,28,36). Organ ischemia below the occlusion is inevitable. Especially after the REBOA procedure, distal perfusion of the lower extremities and organ damage should be monitored because patients with profound and prolonged shock following REBOA are more susceptible to multiple organ failure due to prolonged ischemic time. Reva et al. (43) reported that the REBOA procedure with 60 min of occlusion resulted in significant organ damage, such as acute tubular necrosis, in an ovine model. After deflation of the balloon, when reperfusion is started, a post-reperfusion syndrome, such as generation of inflammatory mediators and free radicals, electrolyte imbalance, and rhabdomyolysis, can occur. To prevent perfusion-related complications, studies on pREBOA or iREBOA should be performed going forward and further devices for distal perfusion should be developed.
Conclusions
REBOA is not a definitive method, but a bridging procedure to achieve hemorrhage control. Therefore, REBOA followed by damage control intervention, such as embolization or laparotomy, should be performed as soon as possible. Cooperation between emergency medical doctors, trauma surgeons, vascular surgeons, and interventional radiologists is necessary to achieve procedures. Currently, the feasibility and safety of REBOA have been investigated in many countries and regions using different registries, such as the AORTA registry (the USA), Aortic Balloon Occlusion in Trauma (ABOTrauma) registry (Europe), the Diagnostic and Interventional Radiology in Emergency, Critical Care and Trauma-Intra-Aortic Balloon Occlusion (DIRECT-IABO) registry (Japan), and Group for Resuscitative Endovascular Advanced treatment on Trauma (GREAT) registry (South Korea).These efforts could contribute to understanding REBOA, and as a result, the survival of hemodynamically unstable patients with NCTH may be improved.
Acknowledgements
The present research was conducted by the research fund of Dankook University in 2018.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Andres J, Scott J, Giannoudis PV. Resuscitative endovascular balloon occlusion of the aorta (REBOA): What have we learned? Injury 2016;47:2603-5. [Crossref] [PubMed]
- Wang H, Phillips JL, Robinson RD, et al. Predictors of mortality among initially stable adult pelvic trauma patients in the US: Data analysis from the National Trauma Data Bank. Injury 2015;46:2113-7. [Crossref] [PubMed]
- Joseph B, Ibraheem K, Haider AA, et al. Identifying potential utility of resuscitative endovascular balloon occlusion of the aorta: An autopsy study. J Trauma Acute Care Surg 2016;81:S128-32. [Crossref] [PubMed]
- Perkins ZB, Lendrum RA, Brohi K. Resuscitative endovascular balloon occlusion of the aorta: promise, practice, and progress? Curr Opin Crit Care 2016;22:563-71. [PubMed]
- Belenkiy SM, Batchinsky AI, Rasmussen TE, et al. Resuscitative endovascular balloon occlusion of the aorta for hemorrhage control: Past, present, and future. J Trauma Acute Care Surg 2015;79:S236-42. [Crossref] [PubMed]
- Abe T, Uchida M, Nagata I, et al. Resuscitative endovascular balloon occlusion of the aorta versus aortic cross clamping among patients with critical trauma: a nationwide cohort study in Japan. Crit Care 2016;20:400. [Crossref] [PubMed]
- Seamon MJ, Haut ER, Van Arendonk K, et al. An evidence-based approach to patient selection for emergency department thoracotomy: A practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg 2015;79:159-73. [Crossref] [PubMed]
- Manzano Nunez R, Naranjo MP, Foianini E, et al. A meta-analysis of resuscitative endovascular balloon occlusion of the aorta (REBOA) or open aortic cross-clamping by resuscitative thoracotomy in non-compressible torso hemorrhage patients. World J Emerg Surg 2017;12:30. [Crossref] [PubMed]
- DuBose JJ, Scalea TM, Brenner M, et al. The AAST prospective Aortic Occlusion for Resuscitation in Trauma and Acute Care Surgery (AORTA) registry: Data on contemporary utilization and outcomes of aortic occlusion and resuscitative balloon occlusion of the aorta (REBOA). J Trauma Acute Care Surg 2016;81:409-19. [Crossref] [PubMed]
- Moore LJ, Brenner M, Kozar RA, et al. Implementation of resuscitative endovascular balloon occlusion of the aorta as an alternative to resuscitative thoracotomy for noncompressible truncal hemorrhage. J Trauma Acute Care Surg 2015;79:523-30; discussion 530-2. [Crossref] [PubMed]
- Stannard A, Eliason JL, Rasmussen TE. Resuscitative endovascular balloon occlusion of the aorta (REBOA) as an adjunct for hemorrhagic shock. J Trauma 2011;71:1869-72. [Crossref] [PubMed]
- Moore LJ, Martin CD, Harvin JA, et al. Resuscitative endovascular balloon occlusion of the aorta for control of noncompressible truncal hemorrhage in the abdomen and pelvis. Am J Surg 2016;212:1222-30. [Crossref] [PubMed]
- Napolitano LM. Resuscitative Endovascular Balloon Occlusion of the Aorta: Indications, Outcomes, and Training. Crit Care Clin 2017;33:55-70. [Crossref] [PubMed]
- Matsumura Y, Matsumoto J, Kondo H, et al. Partial occlusion, conversion from thoracotomy, undelayed but shorter occlusion: resuscitative endovascular balloon occlusion of the aorta strategy in Japan. Eur J Emerg Med 2018;25:348-54. [Crossref] [PubMed]
- van Oostendorp SE, Tan EC, Geeraedts LM Jr. Prehospital control of life-threatening truncal and junctional haemorrhage is the ultimate challenge in optimizing trauma care; a review of treatment options and their applicability in the civilian trauma setting. Scand J Trauma Resusc Emerg Med 2016;24:110. [Crossref] [PubMed]
- Sadek S, Lockey DJ, Lendrum RA, et al. Resuscitative endovascular balloon occlusion of the aorta (REBOA) in the pre-hospital setting: An additional resuscitation option for uncontrolled catastrophic haemorrhage. Resuscitation 2016;107:135-8. [Crossref] [PubMed]
- Hughes CW. Use of an intra-aortic balloon catheter tamponade for controlling intra-abdominal hemorrhage in man. Surgery 1954;36:65-8. [PubMed]
- Heimbecker RO. An aortic tampon for emergency control of ruptured abdominal aneurysm. Can Med Assoc J 1964;91:1024-5. [PubMed]
- Robicsek F, Daugherty HK, Mullen DC. The elective use of balloon obstruction in aortic surgery. Surgery 1970;68:774-7. [PubMed]
- Horer TM, Skoog P, Pirouzram A, et al. A small case series of aortic balloon occlusion in trauma: lessons learned from its use in ruptured abdominal aortic aneurysms and a brief review. Eur J Trauma Emerg Surg 2016;42:585-92. [Crossref] [PubMed]
- Brenner ML, Moore LJ, DuBose JJ, et al. A clinical series of resuscitative endovascular balloon occlusion of the aorta for hemorrhage control and resuscitation. J Trauma Acute Care Surg 2013;75:506-11. [Crossref] [PubMed]
- Inoue J, Shiraishi A, Yoshiyuki A, et al. Resuscitative endovascular balloon occlusion of the aorta might be dangerous in patients with severe torso trauma: A propensity score analysis. J Trauma Acute Care Surg 2016;80:559-66; discussion 566-7. [Crossref] [PubMed]
- Norii T, Crandall C, Terasaka Y. Survival of severe blunt trauma patients treated with resuscitative endovascular balloon occlusion of the aorta compared with propensity score-adjusted untreated patients. J Trauma Acute Care Surg 2015;78:721-8. [Crossref] [PubMed]
- Morrison JJ, Ross JD, Rasmussen TE, et al. Resuscitative endovascular balloon occlusion of the aorta: a gap analysis of severely injured UK combat casualties. Shock 2014;41:388-93. [Crossref] [PubMed]
- Morrison JJ, Galgon RE, Jansen JO, et al. A systematic review of the use of resuscitative endovascular balloon occlusion of the aorta in the management of hemorrhagic shock. J Trauma Acute Care Surg 2016;80:324-34. [Crossref] [PubMed]
- Biffl WL, Fox CJ, Moore EE. The role of REBOA in the control of exsanguinating torso hemorrhage. J Trauma Acute Care Surg 2015;78:1054-8. [Crossref] [PubMed]
- Martinelli T, Thony F, Declety P, et al. Intra-aortic balloon occlusion to salvage patients with life-threatening hemorrhagic shocks from pelvic fractures. J Trauma 2010;68:942-8. [PubMed]
- Saito N, Matsumoto H, Yagi T, et al. Evaluation of the safety and feasibility of resuscitative endovascular balloon occlusion of the aorta. J Trauma Acute Care Surg 2015;78:897-903; discussion 904. [Crossref] [PubMed]
- Ogura T, Lefor AK, Nakamura M, et al. Ultrasound-Guided Resuscitative Endovascular Balloon Occlusion of the Aorta in the Resuscitation Area. J Emerg Med 2017;52:715-22. [Crossref] [PubMed]
- Holcomb JB, Fox EE, Scalea TM, et al. Current opinion on catheter-based hemorrhage control in trauma patients. J Trauma Acute Care Surg 2014;76:888-93. [Crossref] [PubMed]
- Matsumoto J, Lohman BD, Morimoto K, et al. Damage control interventional radiology (DCIR) in prompt and rapid endovascular strategies in trauma occasions (PRESTO): A new paradigm. Diagn Interv Imaging 2015;96:687-91. [Crossref] [PubMed]
- Kim DH, Chang SW. Resuscitative Endovascular Balloon Occlusion of the Aorta: Focusing on the Procedure. Trauma Image Proced 2017;2:92-3. [Crossref]
- Pezy P, Flaris AN, Prat NJ, et al. Fixed-Distance Model for Balloon Placement During Fluoroscopy-Free Resuscitative Endovascular Balloon Occlusion of the Aorta in a Civilian Population. JAMA Surg 2017;152:351-8. [Crossref] [PubMed]
- Qasim Z, Brenner M, Menaker J, et al. Resuscitative endovascular balloon occlusion of the aorta. Resuscitation 2015;96:275-9. [Crossref] [PubMed]
- Johnson MA, Neff LP, Williams TK, et al. Partial resuscitative balloon occlusion of the aorta (P-REBOA): Clinical technique and rationale. J Trauma Acute Care Surg 2016;81:S133-7. [Crossref] [PubMed]
- Low RB, Longmore W, Rubinstein R, et al. Preliminary report on the use of the Percluder occluding aortic balloon in human beings. Ann Emerg Med 1986;15:1466-9. [Crossref] [PubMed]
- Ogura T, Lefor AT, Nakano M, et al. Nonoperative management of hemodynamically unstable abdominal trauma patients with angioembolization and resuscitative endovascular balloon occlusion of the aorta. J Trauma Acute Care Surg 2015;78:132-5. [Crossref] [PubMed]
- Tsurukiri J, Akamine I, Sato T, et al. Resuscitative endovascular balloon occlusion of the aorta for uncontrolled haemorrahgic shock as an adjunct to haemostatic procedures in the acute care setting. Scand J Trauma Resusc Emerg Med 2016;24:13. [Crossref] [PubMed]
- Teeter WA, Matsumoto J, Idoguchi K, et al. Smaller introducer sheaths for REBOA may be associated with fewer complications. J Trauma Acute Care Surg 2016;81:1039-45. [Crossref] [PubMed]
- Gupta BK, Khaneja SC, Flores L, et al. The role of intra-aortic balloon occlusion in penetrating abdominal trauma. J Trauma 1989;29:861-5. [Crossref] [PubMed]
- Irahara T, Sato N, Moroe Y, et al. Retrospective study of the effectiveness of Intra-Aortic Balloon Occlusion (IABO) for traumatic haemorrhagic shock. World J Emerg Surg 2015;10:1. [Crossref] [PubMed]
- Horer TM, Hebron D, Swaid F, et al. Aorta Balloon Occlusion in Trauma: Three Cases Demonstrating Multidisciplinary Approach Already on Patient's Arrival to the Emergency Room. Cardiovasc Intervent Radiol 2016;39:284-9. [Crossref] [PubMed]
- Reva VA, Matsumura Y, Horer T, et al. Resuscitative endovascular balloon occlusion of the aorta: what is the optimum occlusion time in an ovine model of hemorrhagic shock? Eur J Trauma Emerg Surg 2018;44:511-8. [Crossref] [PubMed]


