Cell therapy in acute respiratory distress syndrome
Introduction
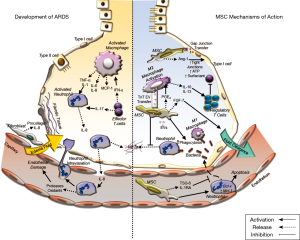
Acute respiratory distress syndrome (ARDS) is a multifactorial syndrome of severe lung injury causing hypoxemia, loss of lung compliance, pulmonary oedema, that can in some instances progress to multiple organ failure (1,2), and results in death in 30–45% of cases (3). ARDS occurs in 10% of all ICU patients and in 23% of all mechanically ventilated patients, with 5.5 ARDS cases per ICU bed each year globally (4). ARDS can develop in response to multiple predisposing factors including pneumonia, systemic infection, and major surgery or multiple traumas (5). This pathology is strongly associated with pulmonary sepsis and/or with a disordered immune response to a major insult (6). Severe lung inflammation is perpetrated by an invasion of neutrophils and macrophages into the alveolar space, which together with the production of pro-inflammatory cytokines, such as interleukin (IL)-6, IL-1β, IL-8 and tumour necrosis factor-alpha (TNF-α), results in damage to the endothelial and epithelial lung layers (7). This inflammatory environment enhances the production of reactive oxygen species, impairs lung barrier function and increases vascular permeability, and, where ARDS is prolonged or unresolved, it can lead to fibrosis (7) (Figure 1).
Current therapies and their shortfalls
There are no direct therapies for ARDS currently, while management strategies such as protective mechanical ventilation and fluid-restrictive strategies minimize iatrogenic harm while providing organ support. Patients with ARDS by definition are extremely hypoxic and require mechanical ventilation, which can exacerbate the acute lung injury (ALI). This is commonly caused by the initial volutrauma that intensifies the inflammatory response and is known clinically as ventilator-induced lung injury (VILI) (8). In ARDS which has developed from a pulmonary or systemic infection, early broad-spectrum antibiotics and source control where possible, are the treatment of choice. Antibiotic therapy presents a low success rate in the treatment of ARDS due to the ongoing inflammatory response which continues to cause injury even after eradication of the pathogen. The emergence of antibiotic-resistant strains and the timing of antibiotic administration can also contribute to this low success (9). Pharmacologic treatments, including glucocorticoids, surfactants, inhaled nitric oxide, antioxidants, protease inhibitors, and a variety of other anti-inflammatory treatments have been tested in clinical trials (10). Unfortunately, these pharmacologic treatments have proven to be completely ineffective (11). In contrast, protective lung ventilation strategies (low tidal volume or limited driving pressure strategy) are currently the accepted gold standard for improving mortality rates in ARDS.
Rationale for cell-based therapies for ARDS
There is a pressing need for a safe and effective treatment for ARDS and attention has turned to the use of cell therapy. The first successful use of cell therapy involved the use of bone marrow (BM) aspirates in a transplant procedure for leukaemia patients (12,13). Originally thought to be the definitive solution for the replacement of damaged tissues by differentiating to replace the damaged cells, further investigations have highlighted that in fact this is not a major mechanism of adult stromal/stem cell action. However, other investigations have highlighted an array of capabilities that some of these cells possess. Their immune-modulating effects, anti-bacterial action, lack of rejection molecules as well as relative ease of isolation and characterisation make these cells an ideal therapeutic for ARDS. In fact, several different cell types have been examined for therapeutic potential.
Stem cell candidates for ARDS
Embryonic stem cells (ESCs)
ESCs are pluripotent cells derived from the inner blastocyst cell mass of the developing embryo (14).These cells can differentiate into all other progenitor cell types (15,16) and their capacity for self-renewal makes them a viable treatment option for tissue regeneration. Studies using human ESCs have shown efficacy in a number of disease models including diabetes mellitus (17), Parkinson’s disease (18), ischemic stroke (19) and some have progressed to clinical trial (20,21). A study by Wang et al., observed that ESC- derived alveolar-epithelial type II cells (AECII) attenuated bleomycin-induced lung injury in mice and thus showed potential promise as a therapeutic (22). There are ethical concerns with the use of this cell type however, and in many countries their use is limited or banned. Another major limiting factor is the safety concerns of ESC-based therapy. The pluripotency of ESCs is a double-edged sword; the same plasticity that permits ESCs to generate hundreds of different cell types also makes them difficult to control after in vivo transplantation, with one of the definitions of ESCs being that after implantation they form teratomas containing cells from all three primary germ layers (23).
Induced pluripotent stem cells (iPSCs)
iPSCs are originally somatic cells of animal or human origin that undergo an induced differentiation treatment, resulting in the overexpression of Oct3/4, Sox2, Klf-4 and c-Myc transcription factors that licence pluripotency (24). iPSCs solve the ethical concerns of ESCs, retaining plasticity and also allowing for autologous transplants. However, iPSCs still present the risk of teratoma formation, for example c-Myc activity has been linked to tumorigenesis (25) while mutagenesis may occur due to the use of lentivirus and adenovirus during the reprogramming process (26). Recent studies have focused on identifying new molecular strategies that can increase cell reprogramming efficiency and that avoid the use of viral transduction (27). A recent study showed that iPSCs significantly alleviated histological damage and cell leakage in a murine model of endotoxin-induced lung injury (28). There are several phase I clinical trials using iPSCs in the treatment of Leukemia (NCT02564484), chronic granulomatous disease (NCT02926963) and retinoblastoma (NCT02193724) for example. iPSCs represent a promising strategy for the therapeutic use of a pluripotent cell type, however much research remains to be conducted to ascertain the safety and enhanced benefits (if any) of these cells over multipotent stem cells.
Mesenchymal stromal/stem cells
MSCs are multipotent adult progenitor cells that can be isolated from numerous sources, including BM, umbilical cord (UC) and adipose tissue (AD), and can be differentiated into mesenchymal lineage cells (29). MSCs are considered to be hypoimmunogenic because they exhibit low levels of MHC-I expression, and no expression of either MHC class II markers or costimulatory molecules, which allows them to avoid immunosurveillance (30) and thus allows allogenic and autologous transplantation (31,32). MSCs have already shown therapeutic efficacy in preclinical models and exhibited safety clinically in a number of phase I trials. Their therapeutic potential, low immunogenicity, ease of harvest and isolation, and low production costs compared with other stem cells have made them the focus of research and consequently, the rest of this review.
While MSCs are traditionally isolated from BM, they can also been found in many other adult tissues such as lung, liver, cord blood, placenta, dental pulp and AD (33), providing alternative, more readily available and cheaper sources of MSCs. These cells have some common morphological and immunophenotypic properties and studies have shown that MSCs derived from UC and AD tissue among others have demonstrated therapeutic efficacy in pre-clinical models of ARDS (34-36). It was recently demonstrated that UC-MSCs could protect against LPS-induced lung injury in a mouse model, with examination of the MSC secretome and identification of factors responsible for the immune regulation leading to a beneficial outcome (37). A study using human AD-MSCs in a mouse model of bleomycin-induced pneumonia has also shown these cells to play a role in immune regulation whereby they reduce the production of pro-inflammatory cytokines and also reduce the proliferation and differentiation of Th2-type CD4+ T-cells, the major T-cell population involved in inflammation (38). The most recent and relevant research studies using MSCs from different tissues are shown in Table 1.
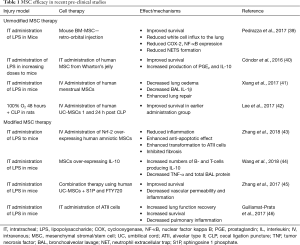
Full table
Progenitor cell candidates for ARDS
Progenitor cells are tissue-specific cells with a limited differentiation capacity, distinguishing them from stem cells. They are found in most tissues and can differentiate and replace injured/damaged cells within organ systems. These cells reside within specific tissue/organ niches; accordingly preclinical studies suggest their therapeutic potential for disease conditions relating to their source tissue, having properties most associated with the repair and regeneration of that tissue (47).
Pulmonary epithelial progenitor cells (EpPCs)
EpPCs have the potential to be used as a therapy in pulmonary diseases both as a direct treatment and also as a potential target in vivo due to their involvement and disruption in certain syndromes (48). A Wnt-responsive alveolar epithelial progenitor cell population expressing AECII surface markers has been recently demonstrated to enhance lung alveoli regeneration in a mouse model of influenza (49).
AEC-IIs, the pulmonary surfactant-producing cells of the lung (48), are a sub-population of EpPCs and their therapeutic potential stems from their ability to rapidly differentiate to AEC-Is, which regulate and control the fluid homeostasis in the alveolar wall and express diverse ion and water channels, and tight junction proteins (50). Intratracheal administration of AEC-IIs aided lung repair through AEC-I transformation and regulated the immune response by synthesizing surfactant and other anti-inflammatory proteins and lipids such as prostaglandin E2 (PGE2) and surfactant protein A (SPA) in a rodent LPS injury model (46). However, the isolation of these cells can be difficult, and the number of cells obtained low.
Endothelial progenitor cells (EnPCs)
EnPCs are circulating cells and their role, isolation and identification has not been fully elucidated. EnPCs express the surface marker CD34 (hematopoietic marker) and seem to have a pivotal role in the repair of the endothelium, adhering to it and other areas under hypoxia or ischemia, releasing growth factors that induce angiogenesis (51,52). One study showed that autologous transplantation of EnPCs improved endothelial function and ameliorated pulmonary oedema following oleic acid- induced ALI in rabbits (53), while another study observed that higher counts of circulating EnPCs correlated to higher survival rates in patients with ALI (54,55). These cells have also shown potential therapeutic use for vascular diseases such as pulmonary arterial hypertension (PAH) as demonstrated during a clinical study by Zhu and colleagues (56). A more recent study has examined the effects of EnPCs transfected to express endothelial nitric oxide synthase in patients with PAH (57). This phase I trial demonstrated the therapeutic potential and safety of this treatment, however further investigations are required to understand the exact mechanism of action of these cells.
Effects of MSCs on the immune system in ARDS
Modulation of the inflammatory response
Cytokine networks between immune and non-immune cells of the alveolar-capillary membrane are necessary for cellular communication during pulmonary inflammation. The subsequent events of these cellular/humoral interactions are pivotal to the initiation and propagation of the inflammatory response leading to pulmonary injury (58). Several studies demonstrate a reduction of the pro-inflammatory cytokines (IL-1α, -1β, -6, -12, -17, TNF-α, TNF-γ and IFN-γ) and an increase in the concentration of anti-inflammatory cytokines and molecules (IL-1 receptor antagonist, IL-10, cyclooxygenase-2 and PGE2) in the lung environment after MSC treatment (41,59-65). Miao et al., demonstrated that MSCs can regulate the NLRP3 inflammasome which regulates the activation of caspase-1 and a subsequent inflammatory response to infectious microbes and molecules in Kupffer cells via secretion of PGE2, leading to increased Kupffer cell production of IL-10. This ameliorated the inflammatory response and ensuing organ dysfunction (66).
Effects on neutrophil response
Upon infection, a series of chemical signals are released, which induce the activation and recruitment of neutrophils to the site of injury (67). Neutrophils kill microorganisms that cause the infection via: phagocytosis, the release of antibacterial peptides, and by creating neutrophil extracellular traps (NETs) (67). If the infection or injury is not resolved, over-stimulation of neutrophils causes the overproduction of inflammatory cytokines at the site of injury (68), thus leading to more damage than resolution (69).
Neutrophils can also migrate from inflamed tissues to other tissues and organ systems causing widespread host injury and organ dysfunction (69). NETs are structures released from neutrophils comprising a core of chromatin DNA and histones, surrounded by specific antimicrobial proteins (lactoferrin, cathepsin G, defensins, LL-37, and bacterial permeability increasing protein), proteases (neutrophil elastase, proteinase-3, and gelatinase), and reactive oxygen species-generating enzymes (myeloperoxidase) (67). However, excessive increases in the release of NETs can also cause damage to lung tissue. Pedrazza et al. demonstrated that MSC treatment enhanced survival in a LPS injury model by reducing NETs, formation (39,40). Numerous pre-clinical ARDS and sepsis studies have shown that MSCs reduce the infiltration of neutrophils to the damaged tissue (64,70) while also enhancing neutrophil-mediated phagocytosis and thus bacterial clearance (71). Németh et al. showed that PGE2 released by MSCs increases the production of IL-10, reducing neutrophil trans-endothelial migration, protecting the organ function and reducing pathogen load (64).
Effects on macrophages
Macrophages are present in almost all tissues, where they coordinate developmental, metabolic, and immunologic functions and thus contribute to the maintenance of homeostasis (72). Upon activation, macrophages develop into two broad phenotypes: M1 or pro-inflammatory macrophages are involved in initiating and sustaining inflammation in response to injury or infection and are required for bacterial killing and clearance, and M2 macrophages, involved in the clearance of dead/injured host cells and tissue repair and immune resolution (73,74). Thus, they play a pivotal role in most aspects of pathologies occurring in the lung. Research has focused on the ability of MSCs to modulate macrophage function by inducing their differentiation to different phenotypes (75,76). Studies suggest that MSCs favour the differentiation of macrophages to the M2 phenotype thus improving the resolution of inflammation and enhancing repair while MSC promotion of the M1 phenotype leads to enhanced phagocytic activity (59,77-79).
Effects on the T-cell response
Regulatory T-cells are a subpopulation of T-cells that modulate the immune system, maintaining self-antigen tolerance and preventing autoimmune disease (80). MSCs promote regulatory T-cell expansion, which causes the suppression of the proliferation of effector T-cells and dampens the immune response, potentially providing a mechanism by which MSCs may enhance ARDS resolution (80). Furthermore, MSCs can modify T-cells, dendritic cells, and natural killer cells, decreasing pro-inflammatory cytokine release and enhancing anti-inflammatory molecule release (81). These effects may be direct or may occur indirectly via effects on dendritic cells and/or other antigen presenting cells (82).
Therapeutic efficacy of MSCs in pre-clinical models of ARDS
As previously described, MSCs offer therapeutic promise for ARDS for several reasons including their immunomodulating ability, reprogramming the immune system to reduce host tissue damage while preserving the immune response to microorganisms and also their capacity to enhance tissue repair after lung injury (83). A study demonstrated that in a septic cecal ligation and puncture (CLP) murine model, MSC therapy modulates transcription of up to 13% of the genome, with immune response–related effects including; down-regulation of toll-like receptor and nuclear factor-κB (NF-κB) activation, a decrease in IL-6 signalling pathways, up-regulation of nuclear factor of activated T-cell (NFAT)-related genes, and genes involved in antigen presentation and cell-to-cell interactions which regulates endothelial integrity, increased phagocytosis and bacterial killing, decreased complement activation, and coagulation regulation including platelet activation (58,84). Furthermore, the long-term effects of MSCs are mitigated by the fact that they disappear from the tissue within days of administration.
Means by which MSCs exert effects
Cell-to-cell contact mediated effects
MSCs can migrate to the damaged lung, and without the need to engraft in the tissue, perform their antimicrobial and tissue repair functions, residing in the tissue for a limited time (85). Liu et al. demonstrated that in ALI, MSCs migrate to the lung, reducing inflammation through direct cell-cell contact (86). Specifically, MSCs showed enhanced therapeutic efficacy after pulmonary lung injury (LPS) versus extra-pulmonary lung injury (LPS/zymosan) due to greater cell recruitment to the lung (86). Islam et al. demonstrated that MSCs in the lung transfer cellular products, including mitochondria via gap junctions to epithelial cells, elevating the ATP levels and improving their function and survival (87). Recently, Jackson et al. demonstrated that MSCs conducted a transfer of mitochondria to macrophages in EVs, via cell-cell contact through tunnelling nanotubules (TnTs), which induced the transformation of these macrophages to a highly phagocytic phenotype and ameliorated E. coli-induced lung injury in vivo (88).
Soluble MSC secretome
Numerous studies have shown that MSCs exert part of their therapeutic efficacy via paracrine mechanisms through the release of an array of soluble molecules known as the ‘MSC secretome’. Curley et al. demonstrated that MSC conditioned medium (MSC-CM) containing the MSC secretome attenuated injury and enhanced repair in a VILI rat model partly by a keratinocyte growth factor (KGF)-dependent mechanism (63). However, Hayes et al. demonstrated in the same animal model, that MSCs produced a better early phase recovery in blood oxygenation and respiratory compliance, and reduction in lung edema when compared to the MSC-CM (89). In another study, MSC-CM caused the down-regulation of inflammatory NF-κB signalling in the lung, which reduced the expression of Bcl-x and Mcl-1 in neutrophils and induced apoptosis of these cells in an endotoxin-induced ALI mouse model (90). In an LPS-induced ALI mouse study it was further demonstrated that MSC-CM produces an improvement in the physiology and histology of the lung (91). Furthermore, this study showed that MSC-CM can induce the differentiation of monocytes to an M2 macrophage phenotype, enhancing the anti-inflammatory and pro-healing environment in part due to the production of insulin-like growth factor (IGF-1) from MSCs (91).
MSC-derived EVs and exosomes
As mentioned previously, MSCs release EVs which incorporate cellular components including mitochondria (87), and gene products such as mRNA and microRNAs (miRNA) (92). Zhu et al. demonstrated that these EVs reduced extravascular lung water and protein levels, decreased pulmonary edema, reduced the alveolar influx of neutrophils, and decreased alveolar macrophage inflammatory protein-2 concentrations after endotoxin-induced ALI in mice (93). Monsel et al. observed that human MSC-derived EVs enhanced survival in a mouse model of E. coli pneumonia, increased ATP levels of epithelial cells, reduced bacterial load, and decreased protein and inflammatory cytokine concentrations—effects that were mediated in part by KGF secretion (92). In recent ARDS research, MSCs were shown to promote an anti-inflammatory and highly phagocytic macrophage phenotype through EV-mediated mitochondrial transfer, reducing lung damage (94,95). Song et al. showed that MSCs stimulated with IL-1β, produce exosomes with high concentration of miR-146a, an anti-inflammatory micro-RNA. This exosomal miR-146a was transferred to macrophages and resulted in M2 polarization. When these exosomes were administered to septic CLP mouse models they lead to an increased survival and were internalised by macrophages in vivo (96). These properties suggest a potential use of stem cell derived EVs for therapy in lung diseases and gives insight to the mechanism of action of MSCs in vivo.
Strategies to enhance MSC therapeutic potential for ARDS
Different methods have been employed to improve the therapeutic effect of MSCs. MSCs treated with poly (I:C), a toll-like receptor-3 ligand, inhibited micro-RNA-143 which increased MSC expression of cyclooxygenase-2, leading to increased PGE2 production and enhanced MSC effects on macrophage function in an in vivo CLP sepsis model (97). Human MSCs overexpressing soluble IL-1 receptor-like-1, the IL-33 antagonist, attenuated endotoxin-induced ALI (65). Recently, Han et al. demonstrated that MSCs transduced with the E-prostanoid 2 receptor enhanced their migration to the injured lung, decreased lung inflammation and reduced endothelial permeability (98). Cai et al. showed that overexpression of orphan receptor tyrosine kinase (ROR2) facilitates MSCs to repair lung injury, enhancing their retention in the lung, and reducing inflammation and pathological impairment (99). MSCs overexpressing the anti-inflammatory cytokine IL-10 resulted in enhanced migration and engraftment, increased wound healing and improved survival rates in a murine model of endotoxin-induced ALI (44).
Another strategy being investigated is the overexpression of proteins that may have a key function in tissue repair. Wang et al. found that human placental MSCs overexpressing platelet-derived growth factor (PDGF) receptor (PDGFR)-β exhibited greater proliferation rates, expressed higher levels of pro-angiogenic factors such as Ang1, VEGF, bFGF and PDGF, and thus enhanced wound repair (100). Overexpression of ANG-1 in MSCs was more effective than naïve MSCs in reducing endotoxin-induced alveolar inflammation and lung permeability (101). Min et al. demonstrated that MSCs overexpressing angiotensin-converting enzyme 2 (ACE2) caused an enhanced reduction in inflammation, and reduced lung edema, collagen deposition and fibrosis after bleomycin-induced lung injury in mice (36). The overexpression of other genes, such as fibroblast growth factor 2 (FGF-2) or KGF have been demonstrated to enhance MSC efficacy in attenuating endotoxin-induced lung injury (102,103).
Other studies have also aimed to improve MSC functionality or longevity; Liu Y et al. showed that inhibition of miRNA-24a enhances survival of BM-MSCs under oxidative stress (104), however the functionality of these MSCs after rescue was not ascertained. Zhang et al., demonstrated that nuclear factor (erythroid-derived 2)-like 2 (Nrf2) transfection of human amniotic mesenchymal stem cells enhances the efficacy of these stem cells to reduce lung damage in an LPS ALI model by decreasing epithelial apoptosis and inflammatory cytokine production (43). Further to this, combination therapies have been investigated to complement and enhance the MSC effect in vivo. The use of a sphingosine 1 phosphate (S1P) analogue FTY720, previously shown to be effective in murine lung injury models (105), administered with UC-MSCs has been demonstrated to yield a better outcome than either treatment alone in terms of mortality and lung injury indices (45). While Chen et al. showed the enhanced effective protection against ARDS with peritoneal sepsis by combined administration of AD-MSCs and pre-activated disaggregated platelets (106). Other substances which may prove beneficial in combination therapy with MSCs include nebulized heparin which has been shown to inhibit coagulation and inflammatory pathways and modulate alveolar macrophages in ALI (107), or using targeting molecules such as glycogen synthase kinase 3 beta inhibitor (GSK-3β) which improves indices of lung injury and promotes the differentiation of MSCs to AT-II cells in in vivo pre-clinical ALI (108)
Progress and challenges
Clinical studies in ARD
Based on their therapeutic promise in pre-clinical studies, MSCs have entered early phase clinical testing in patients with ARDS. Zheng et al. administered allogeneic MSCs to 12 ARDS patients in an intravenous dose of 1 million cells per kilogram, or placebo in a 1:1 ratio (NCT01902082). A study by Wilson et al. included nine patients that received 1, 5, or 10 million cells per kilogram of a single intravenous dose of allogeneic human BM-MSCs, using a three-by-three dose escalation design (NCT01775774). Both studies have shown that MSCs appear to be well tolerated by ARDS patients (Table 2). Zheng et al. also measured IL-6, IL-8, and surfactant protein D levels and found that surfactant protein D concentrations were lower after MSC delivery at day 5 but that MSC administration had no effects on IL-6 or IL-8 levels. There were no significant differences in total length of hospital stay, ICU–free days, and ventilator–free days between treatment arms (Table 2) (109). Another study reported beneficial results of MSCs when administered to two patients in a compassionate use setting (110).
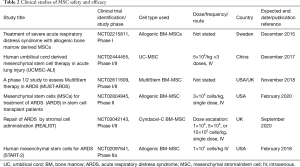
Full table
Barriers to clinical translation
Despite these important advances, several issues must still be investigated before MSCs can be considered as a potential “off the shelf” treatment. Robust assays of MSC batch potency for ARDS are lacking. Factors such as the stage of ARDS, type of MSCs, viability and purity of MSCs, and donor variability, are all poorly understood. The timing of MSC therapy is also relevant, with pre-clinical studies to date generally focused on early MSC delivery. A concern regarding delivery is that during intravenous administration due to the risk of MSC clumping into micro emboli, an obstruction of the pulmonary circulation could occur. The longer-term effects of MSC administration should also be considered with a concern that MSCs could potentially enhance tumorigenesis either by direct malignant transformation or indirectly by facilitating growth of tumor cells, although studies suggest that this is quite unlikely. Frozen cells are often used in studies, as this is necessary for cell transport to clinical sites. In contrast, the majority of preclinical studies use freshly harvested cells. The optimization of cryopreservation strategies for MSCs that maintain cell viability, potency, and efficacy is an important translational challenge.
Commercial production of MSC for the clinical setting
The number of patients receiving MSC based therapies is growing and this increasing demand must be managed legitimately to avoid complications in production and use. The challenges facing the successful marketing of cell therapies has been discussed in several articles (111) which highlight critical hurdles including the scalability, manufacturing and distribution concerns, navigating regulation, cost management, and indeed dealing with the complexity of the cells themselves. In addition to this, focus must be placed on patient safety with regulations needed to avoid exploitation of patients who partake in what is termed ‘stem cell tourism’ and regarding the unlicensed use of stem cell-based therapies (112).
For cell therapy to be viable in patients with critical illnesses, cells must be available within hours and in sufficient numbers from a reproducible production process and be available at relatively low cost. Therefore, there is a need for a strict production process which is highly regulated and licenced by an international authority, however it has been stated that due to complexities of disease states, cell types, and administration procedures, a strict repeatable, reproducible process in cell production may be unfeasible (113). A recent study was conducted to better understand the relationships between commercial and regulatory environments regarding cell-based therapies and products in Canada (114) concluding that there is a ‘reverse governance process’ whereby the regulatory authority relies on the scientific input of researchers and developers to develop the framework. Currently, commercially produced MSCs are manufactured to the companies own (often patented) protocols and are thus the preferred method of MSC acquisition for research rather than ‘in house’ production due to a reduction in variability and an availability of large quantities of cells. However, there are then concerns regarding commercial interests from company entities, the discrepancies in protocols between products, and the ability for research groups to fund and manage clinical trials without vested interests coming into play. The efficacy of MSCs in ARDS has yet to be proven in a large scale clinical trial, requiring the availability of huge quantities of clinical-grade, validated MSCs which would necessitate the involvement of a commercial process. Indeed, there are many issues that need to be addressed to aid in the progress of the development and routine use of cell therapies.
Conclusions
Many challenges remain before MSC therapy becomes the “go to” treatment for ARDS. Further effort is required to optimise their isolation, preparation, and administration in addition to having a better understanding of their mechanism of action. The need for a more abundant source of MSCs is apparent, with UC- and AD-derived MSCs potentially filling this need. Preclinical studies have demonstrated the abundant therapeutic potential of MSCs in ARDS, specifically their capacity to modify the inflammatory response and promote repair. Although MSC treatment seems encouraging in patients in different phase I and II clinical trials, there are still significant hurdles to overcome before these cells can be a truly viable therapy in the clinical setting. The therapeutic efficacy of MSCs can be enhanced by various methods including pre-activation and gene therapy but a complete understanding of their mechanism of action will clarify specific targets to create the most effective phenotype. In understanding the mechanism of action of MSCs, markers of cell potency can be identified, making selection and batch testing more reliable and repeatable, thus ultimately paving the way to an enhanced MSC therapy for the ARDS patient. In an ideal clinical scenario MSCs will constitute a readily available, off the shelf product, with reliable, reproducible effects, that can be tailored to the condition and patient being treated, at an affordable price. In some aspects we are not far from this reality, however some critical hurdles must be overcome to fulfil the criteria of an ‘ideal medicine’.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med 2005;353:1685-93. [Crossref] [PubMed]
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000;342:1334-49. [Crossref] [PubMed]
- Máca J. Past and Present ARDS Mortality Rates: A Systematic Review. Respiratory Care 2017;62:113-22. [Crossref] [PubMed]
- McNicholas BA, Rooney GM, Laffey JG. Lessons to learn from epidemiologic studies in ARDS. Curr Opin Crit Care 2018;24:41-8. [Crossref] [PubMed]
- Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016;315:788-800. [Crossref] [PubMed]
- Martin-Loeches I, Levy MM, Artigas A. Management of severe sepsis: advances, challenges, and current status. Drug Des Devel Ther 2015;9:2079-88. [Crossref] [PubMed]
- Fanelli V, Vlachou A, Ghannadian S, et al. Acute respiratory distress syndrome: new definition, current and future therapeutic options. J Thorac Dis 2013;5:326-34. [PubMed]
- Carrasco Loza R, Villamizar Rodriguez G, Medel Fernandez N. Ventilator-Induced Lung Injury (VILI) in Acute Respiratory Distress Syndrome (ARDS): Volutrauma and Molecular Effects. Open Respir Med J 2015;9:112-9. [Crossref] [PubMed]
- MacArthur RD, Miller M, Albertson T, et al. Adequacy of early empiric antibiotic treatment and survival in severe sepsis: experience from the MONARCS trial. Clin Infect Dis 2004;38:284-8. [Crossref] [PubMed]
- Tonelli AR, Zein J, Adams J, et al. Effects of interventions on survival in acute respiratory distress syndrome: an umbrella review of 159 published randomized trials and 29 meta-analyses. Intensive Care Med 2014;40:769-87. [Crossref] [PubMed]
- Girbes AR, Beishuizen A, Strack van Schijndel RJ. Pharmacological treatment of sepsis. Fundam Clin Pharmacol 2008;22:355-61. [Crossref] [PubMed]
- Thomas ED. Bone marrow transplantation from the personal viewpoint. Int J Hematol 2005;81:89-93. [Crossref] [PubMed]
- Thomas ED, Buckner CD, Banaji M, et al. One hundred patients with acute leukemia treated by chemotherapy, total body irradiation, and allogeneic marrow transplantation. Blood 1977;49:511-33. [PubMed]
- Reubinoff BE, Pera MF, Fong CY, et al. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol 2000;18:399-404. [Crossref] [PubMed]
- Martin GR, Evans MJ. Differentiation of clonal lines of teratocarcinoma cells: formation of embryoid bodies in vitro. Proc Natl Acad Sci U S A 1975;72:1441-5. [Crossref] [PubMed]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science 1998;282:1145-7. [Crossref] [PubMed]
- Soria B, Roche E, Berná G, et al. Insulin-secreting cells derived from embryonic stem cells normalize glycemia in streptozotocin-induced diabetic mice. Diabetes 2000;49:157-62. [Crossref] [PubMed]
- Roy NS, Cleren C, Singh SK, et al. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat Med 2006;12:1259-68. [Crossref] [PubMed]
- Theus MH, Wei L, Cui L, et al. In vitro hypoxic preconditioning of embryonic stem cells as a strategy of promoting cell survival and functional benefits after transplantation into the ischemic rat brain. Exp Neurol 2008;210:656-70. [Crossref] [PubMed]
- Menasché P, Vanneaux V, Hagege A, et al. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: first clinical case report. Eur Heart J 2015;36:2011-7. [Crossref] [PubMed]
- Menasché P, Vanneaux V, Hagège A, et al. Transplantation of Human Embryonic Stem Cell-Derived Cardiovascular Progenitors for Severe Ischemic Left Ventricular Dysfunction. J Am Coll Cardiol 2018;71:429-38. [Crossref] [PubMed]
- Wang D, Morales JE, Calame DG, et al. Transplantation of Human Embryonic Stem Cell Derived Alveolar Epithelial Type II Cells Abrogates Acute Lung Injury in Mice. Mol Ther 2010;18:625-34. [Crossref] [PubMed]
- Nussbaum J, Minami E, Laflamme MA, et al. Transplantation of undifferentiated murine embryonic stem cells in the heart: teratoma formation and immune response. Faseb J 2007;21:1345-57. [Crossref] [PubMed]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663-76. [Crossref] [PubMed]
- Wade M, Wahl GM. c-Myc, Genome Instability, and Tumorigenesis: The Devil Is in the Details. The Myc/Max/Mad Transcription Factor Network. Berlin, Heidelberg: Springer Berlin Heidelberg, 2006:169-203.
- Yoshihara M, Hayashizaki Y, Murakawa Y. Genomic Instability of iPSCs: Challenges Towards Their Clinical Applications. Stem Cell Rev 2017;13:7-16. [Crossref] [PubMed]
- Feng B, Ng JH, Heng JC, et al. Molecules that promote or enhance reprogramming of somatic cells to induced pluripotent stem cells. Cell Stem Cell 2009;4:301-12. [Crossref] [PubMed]
- Yi Fong Su V, Yang KY. Induced Pluripotent Stem Cells Prevent Endothelial Cell Leakage via TIMP-1 to Reduce FAK/SNAIL Pathway in Sepsis-Induced Acute Lung Injury. Respirology 2017;22:60. [Crossref]
- Ding DC, Shyu WC, Lin SZ. Mesenchymal stem cells. Cell Transplant 2011;20:5-14. [Crossref] [PubMed]
- Nasef A, Mathieu N, Chapel A, et al. Immunosuppressive effects of mesenchymal stem cells: involvement of HLA-G. Transplantation 2007;84:231-7. [Crossref] [PubMed]
- Hosseinikia R, Nikbakht MR, Moghaddam AA, et al. Molecular and Cellular Interactions of Allogenic and Autologus Mesenchymal Stem Cells with Innate and Acquired Immunity and Their Role in Regenerative Medicine. Int J Hematol Oncol Stem Cell Res 2017;11:63-77. [PubMed]
- McAuley DF, Curley GF, Hamid UI, et al. Clinical grade allogeneic human mesenchymal stem cells restore alveolar fluid clearance in human lungs rejected for transplantation. Am J Physiol Lung Cell Mol Physiol 2014;306:L809-15. [Crossref] [PubMed]
- Bianco P, Cao X, Frenette PS, et al. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med 2013;19:35-42. [Crossref] [PubMed]
- Kern S, Eichler H, Stoeve J, et al. Comparative Analysis of Mesenchymal Stem Cells from Bone Marrow, Umbilical Cord Blood, or Adipose Tissue. Stem Cells 2006;24:1294-301. [Crossref] [PubMed]
- Mao YX, Xu JF, Seeley Eric J, et al. Adipose Tissue-Derived Mesenchymal Stem Cells Attenuate Pulmonary Infection Caused by Pseudomonas aeruginosa via Inhibiting Overproduction of Prostaglandin E2. Stem Cells 2015;33:2331-42. [Crossref] [PubMed]
- Min F, Gao F, Li Q, et al. Therapeutic effect of human umbilical cord mesenchymal stem cells modified by angiotensin-converting enzyme 2 gene on bleomycin-induced lung fibrosis injury. Mol Med Rep 2015;11:2387-96. [Crossref] [PubMed]
- Zhu H, Xiong Y, Xia Y, et al. Therapeutic Effects of Human Umbilical Cord-Derived Mesenchymal Stem Cells in Acute Lung Injury Mice. Sci Rep 2017;7:39889. [Crossref] [PubMed]
- Kotani T, Masutani R, Suzuka T, et al. Anti-inflammatory and anti-fibrotic effects of intravenous adipose-derived stem cell transplantation in a mouse model of bleomycin-induced interstitial pneumonia. Sci Rep 2017;7:14608. [Crossref] [PubMed]
- Pedrazza L, Cunha AA, Luft C, et al. Mesenchymal stem cells improves survival in LPS-induced acute lung injury acting through inhibition of NETs formation. J Cell Physiol 2017;232:3552-64. [Crossref] [PubMed]
- Cóndor JM, Rodrigues CE. Treatment With Human Wharton's Jelly-Derived Mesenchymal Stem Cells Attenuates Sepsis-Induced Kidney Injury, Liver Injury, and Endothelial Dysfunction. Stem Cells Transl Med 2016;5:1048-57. [Crossref] [PubMed]
- Xiang B, Chen L, Wang X, et al. Transplantation of Menstrual Blood-Derived Mesenchymal Stem Cells Promotes the Repair of LPS-Induced Acute Lung Injury. Int J Mol Sci 2017;18. [Crossref] [PubMed]
- Lee FY, Chen KH, Wallace CG, et al. Xenogeneic human umbilical cord-derived mesenchymal stem cells reduce mortality in rats with acute respiratory distress syndrome complicated by sepsis. Oncotarget 2017;8:45626-42. [PubMed]
- Zhang S, Jiang W, Ma L, et al. Nrf2 transfection enhances the efficacy of human amniotic mesenchymal stem cells to repair lung injury induced by lipopolysaccharide. J Cell Biochem 2018;119:1627-36. [Crossref] [PubMed]
- Wang C, Lv D, Zhang X, et al. Interleukin-10-Overexpressing Mesenchymal Stromal Cells Induce a Series of Regulatory Effects in the Inflammatory System and Promote the Survival of Endotoxin-Induced Acute Lung Injury in Mice Model. DNA Cell Biol 2018;37:53-61. [Crossref] [PubMed]
- Zhang Z, Li W, Heng Z, et al. Combination therapy of human umbilical cord mesenchymal stem cells and FTY720 attenuates acute lung injury induced by lipopolysaccharide in a murine model. Oncotarget 2017;8:77407-14. [PubMed]
- Guillamat-Prats R, Puig F, Camprubi-Rimblas M, et al. Intratracheal instillation of alveolar type II cells enhances recovery from acute lung injury in rats. J Heart Lung Transplant 2018;37:782-91. [Crossref] [PubMed]
- Tesche LJ, Gerber DA. Tissue-derived stem and progenitor cells. Stem Cells Int 2010;2010. [Crossref] [PubMed]
- Rock JR, Hogan BL. Epithelial progenitor cells in lung development, maintenance, repair, and disease. Annu Rev Cell Dev Biol 2011;27:493-512. [Crossref] [PubMed]
- Zacharias WJ, Frank DB, Zepp JA, et al. Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature 2018;555:251. [Crossref] [PubMed]
- Kasper M, Barth K. Potential contribution of alveolar epithelial type I cells to pulmonary fibrosis. Biosci Rep 2017.37. [PubMed]
- Timmermans F, Plum J, Yoder MC, et al. Endothelial progenitor cells: identity defined? J Cell Mol Med 2009;13:87-102. [Crossref] [PubMed]
- Yoder MC. Human endothelial progenitor cells. Cold Spring Harb Perspect Med 2012;2. [Crossref] [PubMed]
- Lam CF, Liu YC, Hsu JK, et al. Autologous transplantation of endothelial progenitor cells attenuates acute lung injury in rabbits. Anesthesiology 2008;108:392-401. [Crossref] [PubMed]
- Burnham EL, Taylor WR, Quyyumi AA, Rojas M, Brigham KL, Moss M. Increased circulating endothelial progenitor cells are associated with survival in acute lung injury. Am J Respir Crit Care Med 2005;172:854-60. [Crossref] [PubMed]
- Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526-33. [PubMed]
- Zhu JH, Xing Xiang W, Fu Rong Z, et al. Safety and efficacy of autologous endothelial progenitor cells transplantation in children with idiopathic pulmonary arterial hypertension: Open†label pilot study. Pediatr Transplant 2008;12:650-5. [Crossref] [PubMed]
- Granton J, Langleben D, Kutryk MB, et al. Endothelial NO-Synthase Gene-Enhanced Progenitor Cell Therapy for Pulmonary Arterial Hypertension. Circ Res 2015;117:645-54. [Crossref] [PubMed]
- Strieter RM, Kunkel SL. Acute lung injury: the role of cytokines in the elicitation of neutrophils. J Investig Med 1994;42:640-51. [PubMed]
- Mei SH, Haitsma JJ, Dos Santos CC, et al. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med 2010;182:1047-57. [Crossref] [PubMed]
- Gupta N, Su X, Popov B, et al. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol 2007;179:1855-63. [Crossref] [PubMed]
- Shin S, Kim Y, Jeong S, et al. The therapeutic effect of human adult stem cells derived from adipose tissue in endotoxemic rat model. Int J Med Sci 2013;10:8-18. [Crossref] [PubMed]
- Chao YH, Wu HP, Wu KH, et al. An increase in CD3+CD4+CD25+ regulatory T cells after administration of umbilical cord-derived mesenchymal stem cells during sepsis. PLoS One 2014;9. [Crossref] [PubMed]
- Curley GF, Ansari B, Hayes M, et al. Effects of intratracheal mesenchymal stromal cell therapy during recovery and resolution after ventilator-induced lung injury. Anesthesiology 2013;118:924-32. [Crossref] [PubMed]
- Németh K, Leelahavanichkul A, Yuen PS, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med 2009;15:42-9. [Crossref] [PubMed]
- Martínez-González I, Roca O, Masclans JR, et al. Human mesenchymal stem cells overexpressing the IL-33 antagonist soluble IL-1 receptor-like-1 attenuate endotoxin-induced acute lung injury. Am J Respir Cell Mol Biol 2013;49:552-62. [Crossref] [PubMed]
- Miao CM, Jiang XW, He K, et al. Bone marrow stromal cells attenuate LPS-induced mouse acute liver injury via the prostaglandin E 2-dependent repression of the NLRP3 inflammasome in Kupffer cells. Immunol Lett 2016;179:102-13. [Crossref] [PubMed]
- Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 2013;13:159-75. [Crossref] [PubMed]
- Sônego F, Castanheira FV, Ferreira RG, et al. Paradoxical Roles of the Neutrophil in Sepsis: Protective and Deleterious. Front Immunol 2016;7:155. [Crossref] [PubMed]
- Nourshargh S, Renshaw SA, Imhof BA. Reverse Migration of Neutrophils: Where, When, How, and Why? Trends Immunol 2016;37:273-86. [Crossref] [PubMed]
- Luo CJ, Zhang FJ, Zhang L, et al. Mesenchymal stem cells ameliorate sepsis-associated acute kidney injury in mice. Shock 2014;41:123-9. [Crossref] [PubMed]
- Hall SR, Tsoyi K, Ith B, et al. Mesenchymal stromal cells improve survival during sepsis in the absence of heme oxygenase-1: the importance of neutrophils. Stem Cells 2013;31:397-407. [Crossref] [PubMed]
- Glass CK, Natoli G. Molecular control of activation and priming in macrophages. Nat Immunol 2016;17:26-33. [Crossref] [PubMed]
- Devaney J, Horie S, Masterson C, et al. Human mesenchymal stromal cells decrease the severity of acute lung injury induced by E. coli in the rat. Thorax 2015;70:625-35. [Crossref] [PubMed]
- Luz-Crawford P, Jorgensen C, Djouad F. Mesenchymal Stem Cells Direct the Immunological Fate of Macrophages. Results Probl Cell Differ 2017;62:61-72. [Crossref] [PubMed]
- Wise AF, Williams TM, Kiewiet MB, et al. Human mesenchymal stem cells alter macrophage phenotype and promote regeneration via homing to the kidney following ischemia-reperfusion injury. Am J Physiol Renal Physiol 2014;306:F1222-35. [Crossref] [PubMed]
- Vasandan AB, Jahnavi S, Shashank C, et al. Human Mesenchymal stem cells program macrophage plasticity by altering their metabolic status via a PGE2-dependent mechanism. Sci Rep 2016;6:38308. [Crossref] [PubMed]
- Zullo JA, Nadel EP, Rabadi MM, et al. The Secretome of Hydrogel-Coembedded Endothelial Progenitor Cells and Mesenchymal Stem Cells Instructs Macrophage Polarization in Endotoxemia. Stem Cells Transl Med 2015;4:852-61. [Crossref] [PubMed]
- Guerra AD, Cantu DA, Vecchi JT, et al. Mesenchymal Stromal/Stem Cell and Minocycline-Loaded Hydrogels Inhibit the Growth of Staphylococcus aureus that Evades Immunomodulation of Blood-Derived Leukocytes. Aaps J 2015;17:620-30. [Crossref] [PubMed]
- Elman JS, Li M, Wang F, et al. A comparison of adipose and bone marrow-derived mesenchymal stromal cell secreted factors in the treatment of systemic inflammation. J Inflamm (Lond) 2014;11:1. [Crossref] [PubMed]
- Belkaid Y, Tarbell K. Regulatory T cells in the control of host-microorganism interactions Annu Rev Immunol 2009;27:551-89. [Crossref] [PubMed]
- Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005;105:1815-22. [Crossref] [PubMed]
- Duffy MM, Ritter T, Ceredig R, et al. Mesenchymal stem cell effects on T-cell effector pathways. Stem Cell Res Ther 2011;2:34. [Crossref] [PubMed]
- Goolaerts A, Pellan-Randrianarison N, Larghero J, et al. Conditioned media from mesenchymal stromal cells restore sodium transport and preserve epithelial permeability in an in vitro model of acute alveolar injury. Am J Physiol Lung Cell Mol Physiol 2014;306:L975-85. [Crossref] [PubMed]
- dos Santos CC, Murthy S, Hu P, et al. Network analysis of transcriptional responses induced by mesenchymal stem cell treatment of experimental sepsis. Am J Pathol 2012;181:1681-92. [Crossref] [PubMed]
- Xu G, Zhang L, Ren G, et al. Immunosuppressive properties of cloned bone marrow mesenchymal stem cells. Cell Res 2007;17:240-8. [Crossref] [PubMed]
- Liu L, He H, Liu A, et al. Therapeutic Effects of Bone Marrow-Derived Mesenchymal Stem Cells in Models of Pulmonary and Extrapulmonary Acute Lung Injury. Cell Transplant 2015;24:2629-42. [Crossref] [PubMed]
- Islam MN, Das SR, Emin MT, et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med 2012;18:759-65. [Crossref] [PubMed]
- Jackson MV, Morrison TJ, Doherty DF, et al. Mitochondrial Transfer via Tunneling Nanotubes is an Important Mechanism by Which Mesenchymal Stem Cells Enhance Macrophage Phagocytosis in the In Vitro and In Vivo Models of ARDS. Stem Cells 2016;34:2210-23. [Crossref] [PubMed]
- Hayes M, Curley GF, Masterson C, et al. Mesenchymal stromal cells are more effective than the MSC secretome in diminishing injury and enhancing recovery following ventilator-induced lung injury. Intensive Care Med Exp 2015;3:29. [Crossref] [PubMed]
- Su VY, Yang KY. Mesenchymal stem cell-conditioned medium induces neutrophils apoptosis via inhibition of NF-kB pathway and increases endogenous pulmonary stem cells in endotoxin-induced acute lung injury. Eur Respir J 2015;46.
- Ionescu L, Byrne RN, van Haaften T, et al. Stem cell conditioned medium improves acute lung injury in mice: in vivo evidence for stem cell paracrine action. Am J Physiol Lung Cell Mol Physiol 2012;303:L967-77. [Crossref] [PubMed]
- Monsel A, Zhu YG, Gennai S, et al. Therapeutic Effects of Human Mesenchymal Stem Cell-derived Microvesicles in Severe Pneumonia in Mice. Am J Respir Crit Care Med 2015;192:324-36. [Crossref] [PubMed]
- Zhu YG, Feng XM, Abbott J, et al. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells 2014;32:116-25. [Crossref] [PubMed]
- Matthay MA. Extracellular Vesicle Transfer from Mesenchymal Stromal Cells Modulates Macrophage Function in Acute Lung Injury. Basic Science and Clinical Implications. Am J Respir Crit Care Med 2017;196:1234-6. [Crossref] [PubMed]
- Morrison TJ, Jackson MV, Cunningham EK, et al. Mesenchymal Stromal Cells Modulate Macrophages in Clinically Relevant Lung Injury Models by Extracellular Vesicle Mitochondrial Transfer. Am J Respir Crit Care Med 2017;196:1275-86. [Crossref] [PubMed]
- Song Y, Dou H, Li X, et al. Exosomal miR-146a Contributes to the Enhanced Therapeutic Efficacy of Interleukin-1beta-Primed Mesenchymal Stem Cells Against Sepsis. Stem Cells 2017;35:1208-21. [Crossref] [PubMed]
- Zhao X, Liu D, Gong W, et al. The toll-like receptor 3 ligand, poly(I:C), improves immunosuppressive function and therapeutic effect of mesenchymal stem cells on sepsis via inhibiting MiR-143. Stem Cells 2014;32:521-33. [Crossref] [PubMed]
- Han J, Lu X, Zou L, et al. E-Prostanoid 2 Receptor Overexpression Promotes Mesenchymal Stem Cell Attenuated Lung Injury. Hum Gene Ther 2016;27:621-30. [Crossref] [PubMed]
- Cai SX, Liu AR, Chen S, et al. The Orphan Receptor Tyrosine Kinase ROR2 Facilitates MSCs to Repair Lung Injury in ARDS Animal Model. Cell Transplant 2016;25:1561-74. [Crossref] [PubMed]
- Wang S, Mo M, Wang J, et al. Platelet-derived growth factor receptor beta identifies mesenchymal stem cells with enhanced engraftment to tissue injury and pro-angiogenic property. Cell Mol Life Sci 2018;75:547-61. [Crossref] [PubMed]
- Mei SH, McCarter SD, Deng Y, et al. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Med 2007;4. [Crossref] [PubMed]
- He H, Liu L, Chen Q, et al. Mesenchymal Stem Cells Overexpressing Angiotensin-Converting Enzyme 2 Rescue Lipopolysaccharide-Induced Lung Injury. Cell Transplant 2015;24:1699-715. [Crossref] [PubMed]
- Zhao YF, Luo YM, Xiong W, et al. Mesenchymal stem cell-based FGF2 gene therapy for acute lung injury induced by lipopolysaccharide in mice. Eur Rev Med Pharmacol Sci 2015;19:857-65. [PubMed]
- Liu Y, Zhang X, Chen J, et al. Inhibition of mircoRNA-34a Enhances Survival of Human Bone Marrow Mesenchymal Stromal/Stem Cells Under Oxidative Stress. Med Sci Monit 2018;24:264-71. [Crossref] [PubMed]
- Natarajan V, Dudek SM, Jacobson JR, et al. Sphingosine-1-phosphate, FTY720, and sphingosine-1-phosphate receptors in the pathobiology of acute lung injury. Am J Respir Cell Mol Biol 2013;49:6-17. [Crossref] [PubMed]
- Chen CH, Chen YL, Sung PH, et al. Effective protection against acute respiratory distress syndrome/sepsis injury by combined adipose-derived mesenchymal stem cells and preactivated disaggregated platelets. Oncotarget 2017;8:82415-29. [PubMed]
- Chimenti L, Camprubi-Rimblas M, Guillamat-Prats R, et al. Nebulized Heparin Attenuates Pulmonary Coagulopathy and Inflammation through Alveolar Macrophages in a Rat Model of Acute Lung Injury. Thromb Haemost 2017;117:2125-34. [Crossref] [PubMed]
- Ding Q, Liu G, Zeng Y, et al. Glycogen synthase kinase3beta inhibitor reduces LPSinduced acute lung injury in mice. Mol Med Rep 2017;16:6715-21. [Crossref] [PubMed]
- Zheng G, Huang L, Tong H, et al. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respir Res 2014;15:39. [Crossref] [PubMed]
- Simonson OE, Mougiakakos D, Heldring N, et al. In Vivo Effects of Mesenchymal Stromal Cells in Two Patients With Severe Acute Respiratory Distress Syndrome. Stem Cells Transl Med 2016;5:845. [Crossref] [PubMed]
- Dodson BP, Levine AD. Challenges in the translation and commercialization of cell therapies. BMC Biotechnology 2015;15:70. [Crossref] [PubMed]
- Heathman TRJ, Nienow AW, McCall MJ, et al. The translation of cell-based therapies: clinical landscape and manufacturing challenges. Regen Med 2015;10:49-64. [Crossref] [PubMed]
- Ginty PJ, Erin AR, Paul H, et al. Regenerative medicine, resource and regulation: lessons learned from the remedi project. Regen Med 2011;6:241-53. [Crossref] [PubMed]
- Isasi R, Rahimzadeh V, Charlebois K. Uncertainty and innovation: Understanding the role of cell-based manufacturing facilities in shaping regulatory and commercialization environments. Appl Transl Genom 2016;11:27-39. [Crossref] [PubMed]


