Bilateral pulmonary sequestrations mimicking advanced lung malignancy
Introduction
Bilateral pulmonary sequestrations are extremely rare and uncommonly diagnosed during adulthood. It can present with severe symptoms such as hemoptysis. It can also be misdiagnosed as a primary lung malignancy.
Case presentation
A 51-year-old man presented with a 2-month history of a cough productive of clear sputum with occasional streaks of blood. He experienced some breathlessness on exertion. He had no fever, night sweats, anorexia or weight loss. There was no history of contact with pulmonary tuberculosis or travel outside the country. He was a smoker of 30 pack-years. There was no family history of malignancy.
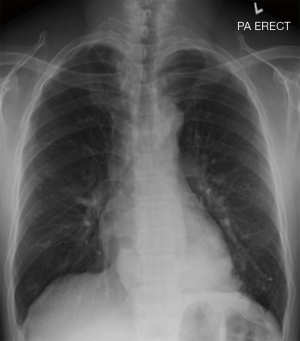
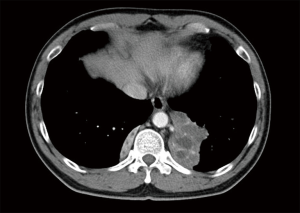
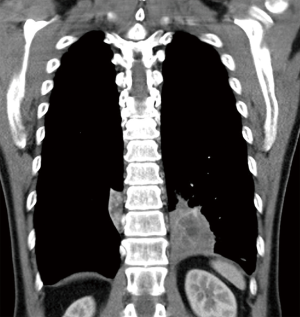
The patient was afebrile with a respiratory rate of 26 breaths/min and oxygen saturation of 100% on room air. Chest auscultation findings were unremarkable. He had mild leucocytosis of 13,700/uL and an elevated C-reactive protein of 9.3 mg/L. His liver function test was deranged with elevated ALT of 110 U/L, AST of 57 U/L and GGT of 739 U/L. Serum carcinoembryonic antigen (CEA) and CA19-9 were elevated at 6.2 ng/mL (normal range, 0.0–2.5 ng/mL) and 478 U/mL (normal range, 0–33 U/mL), respectively. His chest radiograph showed a retrocardiac lung mass (Figure 1). Computed tomography (CT) scan of the thorax revealed two heterogeneous masses at the posterior basal segment of each lower lobe. The left-sided mass was irregular in shape, while the right one was a circumscribed triangular shaped mass within the inferior accessory lobe (Figures 2,3). CT-guided needle biopsy of the retrocardiac mass was complicated by a hemothorax. Histopathological examination of the biopsy specimen revealed features of chronic inflammation with no evidence of malignancy.
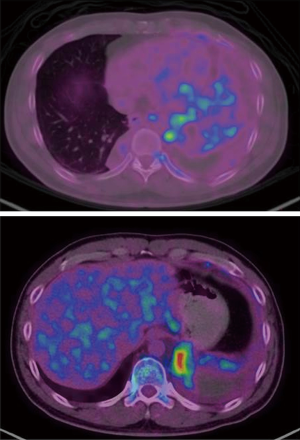
A fluorine-18 (18F-) fluorodeoxyglucose (FDG) positron emission tomography (PET)-CT scan performed subsequently revealed high uptake of 18F-FDG by the left lung mass with a SUVmax of 5.06 while there was no uptake by the right lung mass (Figure 4). There was also increased FDG uptake in the superior mediastinal lymph nodes with a SUVmax of 5.47. A repeat needle biopsy of the left lung mass once again revealed inflamed bronchial tissue and necrosis with no evidence of granuloma or malignancy.
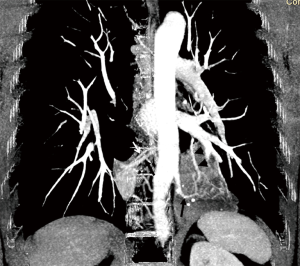
The patient then underwent video-assisted thoracoscopic left lower lobectomy with systematic sampling of the mediastinal lymph nodes. Intraoperatively, a large firm mass in the left lower lobe was found with its arterial supply originating from the descending aorta and draining into the inferior pulmonary vein. Histopathological analysis of the left lower lobe revealed bronchiectatic airways with ongoing chronic inflammation while station 5 and 10 L lymph nodes revealed reactive changes with no evidence of malignancy. Subsequent re-analysis of the maximum intensity projection (MIP) CT images revealed aberrant arterial supply from the lower thoracic aorta to both sequestrated lungs with draining veins to the azygos vein on the right and pulmonary veins on the left, thereby confirming the diagnosis of bilateral pulmonary sequestration (Figure 5).
Discussion
We describe a rare entity of bilateral intralobar pulmonary sequestration, mimicking and misdiagnosed as stage IV lung carcinoma based on clinical symptoms, raised serum CEA, initial radiological findings and 18F-FDG-PET-CT findings, thus subjecting a patient to repeated biopsies, surgery and unwarranted anxiety. A literature search revealed only 5 cases of bilateral intralobar sequestrations reported to date (1,2). In a case series looking at 2,625 cases over a 10-year period in China, up to 21% of pulmonary sequestration cases were misdiagnosed as lung cancer (2).
An oncologic correlation worth mentioning is the relationship between sequestration, and elevated serum levels of CA19-9, as seen in this case. Serum CA19-9 is often elevated in various forms of cancer including choledochal, pancreatic, lung, gastrointestinal and ovarian subtypes. However, serum CA19-9 can be elevated in benign pulmonary disorders including pulmonary sequestration and thus could be used as an additional diagnostic tool (3,4).
A SUVmax cutoff of 2.5 may differentiate benign from malignant pulmonary nodules larger than 1.0 cm in diameter (5). However, in our patient both the left lung lesion and mediastinal lymph nodes were FDG avid with an SUVmax of >2.5. Infected or inflamed intralobar bronchopulmonary sequestrations have been reported to have significantly increased FDG activity and can mimic lung neoplasm on PET-CT (6,7). Non-small cell lung carcinoma has been reported to arise within sequestrations (8,9). In cases with simultaneous involvement of sequestrated lung and lung carcinoma, resection is obviously the mainstay of treatment. Surgical resection should be considered for most patients, especially if they are symptomatic or when cancer cannot be excluded (10).
In the event where diagnosis is uncertain, a careful review of the initial CT especially the reconstructed 3D angiogram should be performed as repeated needle biopsies in misdiagnosing pulmonary sequestration as tumor may result in hemorrhage and hemothorax as a complication as seen with the case described. Moreover, failure to identify the aberrant vessels properly by a careful review of the CT angiogram and during the course of surgical resection can result in inadvertent trauma and catastrophic hemorrhage (11).
Conclusions
Bilateral intralobar pulmonary sequestrations are extremely rare and can be misdiagnosed as advanced lung malignancy. 18F-FDG-PET-CT cannot differentiate the former from the latter. As the hallmark of diagnosis of pulmonary sequestration remains the presence of aberrant vessels to the sequestrated mass, a careful review of the initial CT including reconstructed 3D angiogram is of utmost value in coming to a diagnosis, saving the patient from unwarranted anxiety and catastrophic hemorrhage during a biopsy or resection.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images without any identifying information.
References
- Savic B, Birtel F, Tholen W, et al. Lung sequestration: report of seven cases and review of 540 published cases. Thorax 1979;34:96-101. [Crossref] [PubMed]
- Wei Y, Li F. Pulmonary sequestration: a retrospective analysis of 2625 cases in China. Eur J Cardiothorac Surg 2011;40:e39-42. [Crossref] [PubMed]
- Yagyu H, Adachi H, Furukawa K, et al. Intralobar pulmonary sequestration presenting increased serum CA19-9 and CA 125. Intern Med 2002;41:875-8. [Crossref] [PubMed]
- Matsuoka H, Nohara H. Pulmonary sequestration with high levels of tumor markers tending to be misdiagnosed as lung cancer. Jpn J Thorac Cardiovasc Surg 2006;54:117-9. [Crossref] [PubMed]
- Khalaf M, Abdel-Nabi H, Baker J, et al. Relation between nodule size and 18F-FDG-PET SUV for malignant and benign pulmonary nodules. J Hematol Oncol 2008;1:13. [Crossref] [PubMed]
- Chan VCY, Boiselle PM, Karchmer AW, et al. Infected intralobar bronchopulmonary sequestration mimicking lung neoplasm on CT and positron emission tomography. Am J Roentgenol 2002;179:805. [Crossref]
- Su M, Fan Q, Fan C, et al. Lung sequestration and Pott disease masquerading as primary lung cancer with bone metastases on FDG PET/CT. Clin Nucl Med 2009;34:236-8. [Crossref] [PubMed]
- Simoglou C, Lawal LA. Adenocarcinoma in pulmonary sequestration: A case report and literature review. Asian Cardiovasc Thorac Ann 2015;23:1119-20. [Crossref] [PubMed]
- Lee BF, Chang HY, Yan JJ, et al. Carcinoma of the lung misinterpreted as pulmonary sequestration on contrast CT but correctly identified on FDG PET/CT. Clin Nucl Med 2010;35:343-5. [Crossref] [PubMed]
- Okamoto T, Masuya D, Nakashima T, et al. Successful treatment for lung cancer associated with pulmonary sequestration. Ann Thorac Surg 2005;80:2344-6. [Crossref] [PubMed]
- Kestenholz PB, Schneiter D, Hillinger S, et al. Thorascopic treatment of pulmonary sequestration. Eur J Cardiothorac Surg 2006;29:815-8. [Crossref] [PubMed]