Neutrophil/lymphocyte ratio is helpful for predicting weaning failure: a prospective, observational cohort study
Introduction
Weaning from invasive mechanical ventilation (IMV) remains a huge challenge to critical care physicians (1,2). The weaning process can account for 40–50% of the total duration of IMV, which can delay extubation and lead to complications and/or death (2-4). Although spontaneous breathing trials (SBTs) are reliable tests for judging weaning outcomes, 13% of patients with successful SBT results still require reintubation (2,5). Moreover, roughly 30% of IMV patients experience difficult or prolonged weaning, which can lead to morbidities or even death (2). Hence, it is of significant importance to enhance the accuracy of methods predicting weaning outcome.
The ratio of neutrophils to lymphocytes (NLR) in peripheral blood samples may correlate closely with systematic inflammation and/or stress and has been shown to have promising predictive ability under several clinical circumstances as a simple, inexpensive, and clinically accessible marker (6-21). In critically ill patients, NLR has a robust association with the severity of disease andmortality (7,9). In an emergency care setting, NLR has been found to be more accurate for predicting bacteremia and the severity of disease than other markers (10,11). Among sepsis patients, the ratio is independently correlated with dreadful clinical prognoses (12). Among patients with chronic obstructive pulmonary disease (COPD), it may predict the severity of disease and the potential for exacerbations (13,14). In addition, NLR has been shown to be a valuable prognostic marker for various chronic conditions ranging from oncological to cardiovascular diseases (6,15-21).
Patients would show an inflammatory response when suffering from systematic or local infections and when undergoing endotracheal intubation and subsequent IMV (7,22-24). Moreover, such a response would become more severe in the presence of IMV-related complications such as ventilator-induced lung injury (25). In addition, previous studies (26-28) have shown that patients who fail weaning (WF) experience higher pulmonary and cardiovascular stress than those who are successfully weaned (WS). Hence, patients with signs of such responses might be prime candidates for WF. However, to date, the relationship between inflammation and/or stress and weaning outcome does not appear to have been investigated.
We hypothesized that NLR would be higher in WF patients than in WS patients. Accordingly, we evaluated the utility of NLR for predicting WF in patients receiving IMV, compared to traditional inflammatory markers such as leukocyte counts and levels of C-reactive protein (CRP).
Methods
Study design
We performed an observational prospective cohort study from July 2013 to December 2016 in the 12-bed intensive care unit (ICU) of Beijing Chao-Yang Hospital in China. A total of 269 consecutive patients receiving IMV were enrolled, with closest relatives or other surrogates providing written informed consent. Our hospital’s ethics committee approved our protocol (No. 2013-KE-24), and the study was retrospectively registered at ClinicalTrials.gov (NCT02981589).
Patients
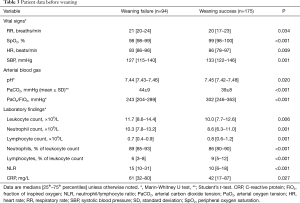
Inclusion and exclusion criteria are shown in Table 1.

Full table
Weaning protocol
All enrolled patients completed a 2 h SBT during which they could breathe spontaneously through a T-tube circuit with the FiO2 adjusted to match each patient’s corresponding IMV setting. During the trial, respiratory rate (RR), systolic blood pressure (SBP), heart rate (HR), pulse oxymetry, five-lead electrocardiographic tracing, and clinical signs were closely monitored. Arterial blood gas measurements were made before and at the very end of SBT. Trials were considered to have failed and were terminated when a patient’s condition worsened and met the SBT failure criteria (see Table 1 for definition) at any time during the 2 h. Otherwise, patients were considered to have passed the SBT and were extubated.
Patients were monitored closely for at least 48 h after extubation. If patients experienced respiratory distress (see Table 1 for definition), rescue noninvasive ventilation (NIV) was considered; if their condition worsened and they met the reintubation criteria (see Table 1 for definition), they were reintubated.
The decision to start SBT, reinstitute IMV during or at the end of SBT, extubate/reintubate patients, or attempt rescue NIV was left to the attending physician, who was blinded to the study.
Data collection and definitions
At enrollment, patients’ baseline characteristics were recorded, including demographic data, acute physiology and chronic health evaluation II (APACHE II) score, IMV duration prior to weaning, underlying diseases, and causes of IMV. Prior to weaning, we recorded vital signs and arterial blood gas data. After extubation, the following was recorded: success or failure of weaning, length of stay (in ICU and hospital), mortality (in ICU and hospital), and 28-day survival.
Peripheral venous blood samples were drawn at the beginning of SBT to measure complete blood cell counts and CRP levels. NLR was determined by dividing the total number of neutrophils by the total number of lymphocytes.
The primary outcome was WF defined as the need for rescue NIV, reintubation <48 h post-extubation, or reinstitution of IMV during or at the very end of SBT (2). Secondary outcomes included length of stay (in ICU and hospital), mortality (in ICU and hospital), and 28-day survival.
Statistical analyses
To compare continuous variables, the Kolmogorov–Smirnov test was used to test the normality of the data, Levene’s test was applied to assess homogeneity of variance, Student’s t-test was used for normally distributed data (expressed as means ± standard deviations), and the Mann-Whitney U test was utilized for non-normally distributed data [expressed as medians (with 25th–75th percentiles)]. The Chi-square test (with/without continuity correction, as needed) was employed to compare qualitative or categorical variables (expressed as absolute values with percentages).
Correlations between NLR and leukocytes/CRP were investigated using Spearman’s correlations, and the results are displayed as correlation coefficients and P values. The utility of each marker (NLR, leukocyte levels, and CRP) for predicting WF was analyzed based on receiver-operating characteristic (ROC) curves. For each ROC curve, we calculated the optimal cutoff values, Youden’s index, sensitivity, specificity, diagnostic accuracy, the area under the curve (AUC, with 95% CIs), positive/negative predictive value, and the likelihood ratio of positive/negative tests.
Next, to investigate the potential associations between each of these markers and WF, we first conducted univariate analyses, which resulted in an odds ratio (95% CI) for each variable. Then, to determine if any of these markers were independently associated with WF, we conducted multivariate analyses applying a conditional forward stepwise model (with an entry level of 0.05 and a removal level of 0.10), which resulted in adjusted odds ratios (95% CIs). These analyses were performed with all possible confounders (e.g., age, gender, chronic respiratory disorders, postoperative respiratory failure, neurological disease, APACHE II score at baseline, and RR, SpO2, HR, SBP, pH, PaCO2, and PaO2/FiO2 prior to weaning) as covariates. Kaplan-Meier 28-day survival curves were constructed according to weaning outcome, NLR, leukocyte counts, and CRP levels; log-rank tests were used to compare the curves.
All analyses were two-tailed, and differences were considered to be statistically significant at P<0.05. The SPSS software package (version 19.0, SPSS, Chicago, IL, USA) was used for all statistical analyses.
Results
Patient characteristics and weaning outcome
A total of 269 patients were included in the study (see Figure 1 for a flow chart of patient selection). Of these, 94 (34.9%) failed the weaning process (66 failed the SBT and 28 presented with post-extubation respiratory distress). Reasons for post-extubation respiratory distress included hypoxemia (n=12), respiratory acidosis (n=10), excess respiratory secretions (n=3), aspiration (n=2), and pulmonary edema (n=1). In 18 of these patients, reintubation was prevented via rescue NIV; the rest were ultimately reintubated.
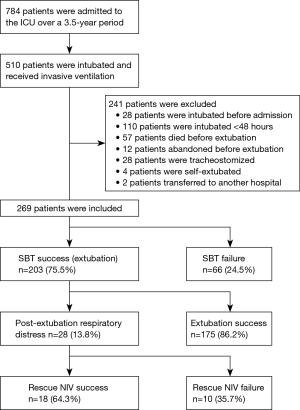
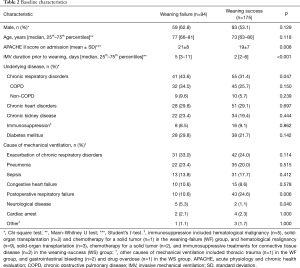
Table 2 summarizes the baseline characteristics of the study population. Compared to WS patients, the WF group had higher rates of chronic respiratory failure and neurological disease; lower rates of postoperative respiratory failure; higher scores on APACHE II; and longer IMV durations prior to weaning. RR, SpO2, HR, SBP, pH, PaCO2, and PaO2/FiO2 prior to weaning significantly differed between the two groups (Table 3).
Full table
Full table
Pre-weaning leukocyte and CRP levels, and NLR
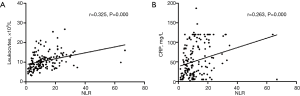
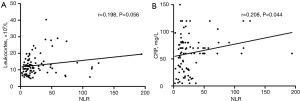
As shown in Figure 2 and Table 3, WF patients had higher NLRs (P<0.001), leukocyte counts (P=0.006), and CRP levels (P=0.027) than WS patients. More specifically, they had higher neutrophil counts (P=0.001), a higher percentage of neutrophils among total leukocyte levels (P<0.001), lower overall lymphocyte counts (P=0.001), and a lower percentage of lymphocytes among total leukocyte levels (P<0.001) (Table 3). There were significant positive correlations between NLR and leukocyte count (rho =0.325, P<0.001) and CRP (rho =0.263, P<0.001) in WS patients (Figure 3), and between NLR and CRP (rho =0.208, P=0.044) in WF patients (Figure 4). NLR and leukocyte count (rho =0.198, P=0.056) also tended to be correlated in WF patients, but the relation did not reach statistical significance (Figure 4).

Predictive ability of NLR, leukocyte counts, and CRP levels for WF
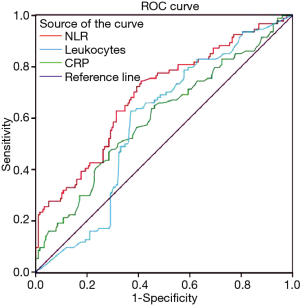
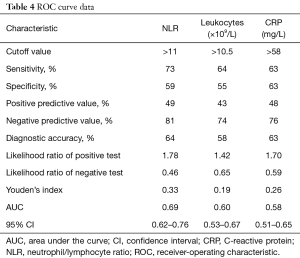
The ROC curves for NLR, leukocytes, and CRP are presented in Figure 5. The AUC of the NLR ROC curve (0.69; 95% CI, 0.62–0.76) was higher than that of leukocytes (0.60; 95% CI, 0.53–0.67) and CRP (0.58; 95% CI, 0.51–0.65). The best combination of cut-off values that resulted in optimal diagnostic accuracy (63%, 58%, and 64%, respectively), positive predictive value (48%, 43%, and 49%), negative predictive value (76%, 74%, and 81%), specificity (63%, 55%, and 59%), and sensitivity (63%, 64%, and 73%) for predicting WF was CRP >58 mg/L, leukocyte counts >10.5×109/L, and NLR >11 prior to weaning (Table 4).
Full table
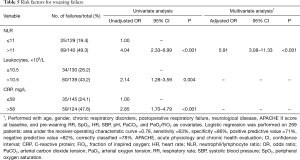
According to univariate analyses, patients with NLR >11, leukocyte counts >10.5×109/L, and CRP >58 mg/L had a higher probability of WF (Table 5). According to multivariate analyses, only NLR >11 was an independent predictor of WF (Table 5).
Full table
Table 6 shows the baseline and weaning characteristics of the patients according to NLR cutoff values. Compared to patients with NLR ≤11, patients with NLR >11 were older and had a longer IMV duration prior to weaning and higher HR but lower pH and rate of postoperative respiratory failure. The WF rate was significantly higher in patients with NLR >11 than in those with NLR ≤11 [69/140 (49%) vs. 25/129 (19%); P<0.001].
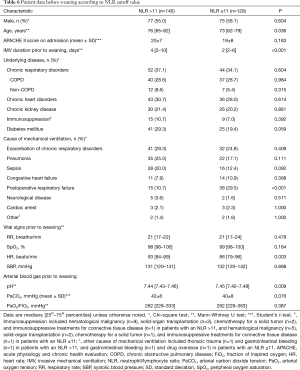
Full table
Patient outcome
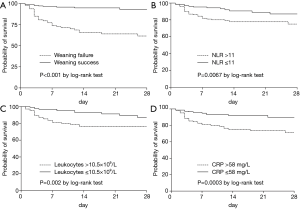
Compared to WS patients, the WF group had higher ICU mortality [33/94 (35%) vs. 18/175 (10%); P<0.001] and hospital mortality [40/94 (43%) vs. 24/175 (14%); P<0.001], and longer length of ICU stay {14 [7–26] vs. 5 [3–16] days; P<0.001}. The two groups did not differ significantly in hospital stay duration {19 [10–40] vs. 16 [11–30] days; P=0.519}. As shown in Figure 6, the cumulative survival probability within 28 days after randomization was lower in patients with WF (P<0.001 by log-rank test), NLR >11 (P=0.0067 by log-rank test), leukocyte counts >10.5 ×109/L (P=0.002 by log-rank test), and CRP >58 mg/L (P=0.0003 by log-rank test).
Discussion
We believe that this study is the first to exclusively explore the potential of NLR for predicting WF, and one of a few studies to investigate the usefulness of NLR in an ICU. Systemic inflammation and/or stress is commonly present during IMV, suggesting that it is worthwhile to elucidate the potential association between such responses and weaning outcome (23,28,29). Our main finding that the discriminatory ability of NLR (a marker of inflammation and/or stress) for WF is superior to that of traditional inflammatory markers makes it clear that NLR may be a useful marker for predicting WF, and thus weaning at higher NLRs might be considered with caution.
WF is typically regarded as when a patient fails SBT or must be reintubated within 48 h after extubation (2,30,31). However, prophylactic and curative NIV are frequently attempted during weaning in an ICU, which would partially avert extubation failure and challenge the traditional definition (32-35). Hence, as recommended by an international consensus conference, we defined WF as SBT failure or the resumption of noninvasive or invasive ventilation within 48 h following extubation (2). Such a pragmatic definition is similar to that of previous studies where NIV was not used, but would be more suitable to current clinical practices in ICUs (30,31).
We found that NLR was higher in WF patients than in WS patients, the AUC of the NLR ROC curve was the highest among the evaluated markers, and NLR >11 was the only independent predictor of WF. There may be an underlying causal effect that can explain these findings. Theoretically, inflammation and/or stress tend to trigger increases in neutrophil levels and decreases in lymphocyte levels, resulting in an increase in NLR (11). Furthermore, neutrophilia is caused by demargination and delayed apoptosis of neutrophils, and stimulation of stem cells by growth factors, whereas in lymphopenia, lymphocytes tend to be marginated and redistributed within the lymphatic system and apoptosis is markedly accelerated (36-39). A more severe inflammatory and/or stress response would result in a higher NLR. Such conditions may be caused by certain unresolved inflammatory diseases that cause respiratory failure and prompt IMV, IMV-related complications, and/or the condition of cardiopulmonary stress on which spontaneous breathing capability becomes limited to counterbalance respiratory load (2,25,28). All of these factors could possibly lead to weaning difficulty (2). Accordingly, there would be an independent association between inflammation and/or stress and WF, and thus NLR might have the potential to help predict WF.
Leukocyte levels, a well-known inflammatory marker, are frequently used in current clinical practice (11). We explored this marker’s ability to predict WF and compared it to NLR. We found that NLR was a more valuable predictor of WF than leukocyte levels. A possible explanation is that leukocytes mainly represent changes in neutrophils, while NLR represents changes in both neutrophils and lymphocytes (11). Hence, NLR may better indicate inflammatory and/or stress responses. In line with this finding, previous studies have reported that NLR is a better predictor of bacteremia and mortality than leukocyte levels during emergency care (10,11). It also appears to be better than leukocyte counts for predicting the prognosis of cardiovascular disease (40).
CRP is also a conventional systemic marker of inflammation, and its plasma concentration increases in response to inflammation (41). Nevertheless, we found that NLR outperformed CRP in predicting WF, in line with previous studies that have reported that CRP has relatively poor predictive ability for bacteremia and mortality in emergency patients compared to NLR (10,11), and is not superior to using a combination of neutrophilia and lymphocytopenia for predicting bacteremia (42).
Limitations
This study had several limitations. First, considering the single-center nature of the study, our findings should be generalized with caution to other settings. Second, although our hospital has established criteria for all clinical procedures and decisions, each attending physician made personal decisions regarding when to start SBT, extubate/reintubate patients, reinstitute IMV during SBT, or attempt rescue NIV; consequently, it is impossible to guarantee that every patient was managed identically throughout the study. Third, neutrophil levels, also a traditional inflammatory maker, were not compared to NLR because the ratio already includes neutrophils; in addition, changes in leukocyte levels mainly represent changes in neutrophils (11). Finally, certain co-morbidities (e.g., malnutrition, hematological disease) would affect the inflammatory responses of circulating white blood cells and even change the NLR, but relevant data were absent; nonetheless, this could confound our findings (43,44). However, it is difficult to obtain all data on patients in an ICU.
Conclusions
This study offers the first experimental data indicating that the discriminatory ability of NLR in WF is superior to that of leukocyte counts and CRP levels, and NLR >11 was an independent predictor of WF. We suggest that NLR may be a useful predictor of WF and that weaning at NLR >11 might be considered with caution. However, further study with a larger sample size is warranted to determine whether NLR as a biomarker for predicting WF would improve weaning outcomes.
Acknowledgements
We thank the medical and nursing team in the intensive care unit of Beijing Chao-Yang Hospital for their assistance during the present study.
Funding: This study was supported by the Nation Natural Science Foundation of China (No.81570070), and the Capital Characteristic Clinical Application Research from Beijing Municipal Science & Technology Commission (No. Z141107002514133).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the ethics committee of Beijing Chao-Yang Hospital (No. 2013-KE-24), and closest relatives or other surrogates provided written informed consent, if appropriate.
References
- MacIntyre NR, Cook DJ, Ely EW, et al. Evidence-based guidelines for weaning and discontinuing ventilatory support: a collective task force facilitated by the American College of Chest Physicians; the American Association for Respiratory Care; and the American College of Critical Care Medicine. Chest 2001;120:375S-95S. [Crossref] [PubMed]
- Boles JM, Bion J, Connors A, et al. Weaning from mechanical ventilation. Eur Respir J 2007;29:1033-56. [Crossref] [PubMed]
- Esteban A, Anzueto A, Frutos F, et al. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. JAMA 2002;287:345-55. [Crossref] [PubMed]
- Esteban A, Ferguson ND, Meade MO, et al. Evolution of mechanical ventilation in response to clinical research. Am J Respir Crit Care Med 2008;177:170-7. [Crossref] [PubMed]
- Ouellette DR, Patel S, Girard TD, et al. Liberation from mechanical ventilation in critically ill adults: an official American College of Chest Physicians/American Thoracic Society clinical practice guideline: inspiratory pressure augmentation during spontaneous breathing trials, protocols minimizing sedation, and noninvasive ventilation immediately after extubation. Chest 2017;151:166-80. [Crossref] [PubMed]
- Kawahara T, Furuya K, Nakamura M, et al. Neutrophil-to-lymphocyte ratio is a prognostic marker in bladder cancer patients after radical cystectomy. BMC Cancer 2016;16:185. [Crossref] [PubMed]
- Zahorec R. Ratio of neutrophil to lymphocyte counts: rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy 2001;102:5-14. [PubMed]
- Sørensen AK, Holmgaard DB, Mygind LH, et al. Neutrophil-to-lymphocyte ratio, calprotectin and YKL-40 in patients with chronic obstructive pulmonary disease: correlations and 5-year mortality – a cohort study. J Inflamm (Lond) 2015;12:20. [Crossref] [PubMed]
- Salciccioli JD, Marshall DC, Pimentel MA, et al. The association between the neutrophil-to-lymphocyte ratio and mortality in critical inllness: an observational cohort study. Crit Care 2015;19:13. [Crossref] [PubMed]
- de Jager CP, van Wijk PT, Mathoera RB, et al. Lymphocytopenia and neutrophil-lymphocyte count ratio predict bacteremia better than conventional infection markers in an emergency care unit. Crit Care 2010;14:R192. [Crossref] [PubMed]
- de Jager CP, Wever PC, Gemen EF, et al. The neutrophil-lymphocyte count ratio in patients with community-acquired pneumonia. PLoS One 2012;7. [Crossref] [PubMed]
- Liu X, Shen Y, Wang H, et al. Prognostic significance of neutrophil-to-lymphocyte ratio in patients with sepsis: a prospective observational study. Mediators Inflamm 2016;2016. [Crossref] [PubMed]
- Günay E, Sarinc Ulasli S, Akar O, et al. Neutrophil-to-lymphocyte ratio in chronic obstructive pulmonary disease: a retrospective study. Inflammation 2014;37:374-80. [Crossref] [PubMed]
- Furutate R, Ishii T, Motegi T, et al. The neutrophil to lymphocyte ratio is related to disease severity and exacerbation in patients with chronic obstructive pulmonary disease. Intern Med 2016;55:223-9. [Crossref] [PubMed]
- Sarraf KM, Belcher E, Raevsky E, et al. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer. J Thorac Cardiovasc Surg 2009;137:425-8. [Crossref] [PubMed]
- Halazun KJ, Hardy MA, Rana AA, et al. Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann Surg 2009;250:141-51. [Crossref] [PubMed]
- Hu K, Lou L, Ye J, et al. Prognostic role of the neutrophil-lymphocyte ratio in renal cell carcinoma: a meta-analysis. BMJ Open 2015;5. [Crossref] [PubMed]
- Paquissi FC. The role of inflammation in cardiovascular diseases: the predictive value of neutrophil-lymphocyte ratio as a marker in peripheral arterial disease. Ther Clin Risk Manag 2016;12:851-60. [Crossref] [PubMed]
- Arbel Y, Finkelstein A, Halkin A, et al. Neutrophil/lymphocyte ratio is related to the severity of coronary artery disease and clinical outcome in patients undergoing angiography. Atherosclerosis 2012;225:456-60. [Crossref] [PubMed]
- Arbel Y, Shacham Y, Ziv-Baran T, et al. Higher neutrophil/lymphocyte ratio is related to lower ejection fraction and higher long-term all-cause mortality in ST-elevation myocardial infarction patients. Can J Cardiol 2014;30:1177-82. [Crossref] [PubMed]
- Akpek M, Kaya MG, Lam YY, et al. Relation of neutrophil/lymphocyte ratio to coronary flow to in-hospital major adverse cardiac events in patients with ST-elevated myocardial infarction undergoing primary coronary intervention. Am J Cardiol 2012;110:621-7. [Crossref] [PubMed]
- Tavares LP, Teixeira MM, Garcia CC. The inflammatory response triggered by Influenza virus: a two edged sword. Inflamm Res 2017;66:283-302. [Crossref] [PubMed]
- Hennus MP, van Vught AJ, Brabander M, et al. Mechanical ventilation drives inflammation in severe viral bronchiolitis. PLoS One 2013;8. [Crossref] [PubMed]
- Machado HS, Nunes CS, Sa P, Couceiro A, et al. Increased lung inflammation with oxygen supplementation in tracheotomized spontaneously breathing rabbits: an experimental prospective randomized study. BMC Anesthesiol 2014;14:86. [Crossref] [PubMed]
- Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med 2013;369:2126-36. [Crossref] [PubMed]
- Jubran A, Tobin MJ. Pathophysiologic basis of acute respiratory distress in patients who fail a trial of weaning from mechanical ventilation. Am J Respir Crit Care Med 1997;155:906-15. [Crossref] [PubMed]
- Jubran A, Mathru M, Dries D, et al. Continuous recordings of mixed venous oxygen saturation during weaning from mechanical ventilation and the ramifications thereof. Am J Respir Crit Care Med 1998;158:1763-9. [Crossref] [PubMed]
- Sellarés J, Loureiro H, Ferrer M, et al. The effect of spontaneous breathing on systemic interleukin-6 during ventilator weaning. Eur Respir J 2012;39:654-60. [Crossref] [PubMed]
- Koksal GM, Sayilgan C, Sen O, et al. The effects of different weaning modes on the endocrine stress response. Crit Care 2004;8:R31-4. [Crossref] [PubMed]
- Esteban A, Frutos F, Tobin MJ, et al. A comparison of four methods of weaning patients from mechanical ventilation. Spanish Lung Failure Collaborative Group. N Engl J Med 1995;332:345-50. [Crossref] [PubMed]
- Vallverdú I, Calaf N, Subirana M, et al. Clinical characteristics, respiratory functional parameters, and outcome of a two-hour T-piece trial in patients weaning from mechanical ventilation. Am J Respir Crit Care Med 1998;158:1855-62. [Crossref] [PubMed]
- Hilbert G, Gruson D, Portel L, et al. Noninvasive pressure support ventilation in COPD patients with postextubation hypercapnic respiratory insufficiency. Eur Respir J 1998;11:1349-53. [Crossref] [PubMed]
- Hess DR. The role of noninvasive ventilation in the ventilator discontinuation process. Respir Care 2012;57:1619-25. [Crossref] [PubMed]
- Ferrer M, Valencia M, Nicolas JM, et al. Early noninvasive ventilation averts extubation failure in patients at risk: a randomized trial. Am J Respir Crit Care Med 2006;173:164-70. [Crossref] [PubMed]
- Ferrer M, Sellares J, Valencia M, et al. Non-invasive ventilation after extubation in hypercapnic patients with chronic respiratory disorders: randomised controlled trial. Lancet 2009;374:1082-8. [Crossref] [PubMed]
- Jilma B, Blann A, Pernerstorfer T, et al. Regulation of adhesion molecules during human endotoxemia: no acute effects of aspirin. Am J Respir Crit Care Med 1999;159:857-63. [Crossref] [PubMed]
- Mahidhara R, Billiar TR. Apoptosis in sepsis. Crit Care Med 2000;28:N105-13.
- Le Tulzo Y, Pangault C, Gacouin A, et al. Early circulating lymphocyte apoptosis in human septic shock is associated with poor outcome. Shock 2002;18:487-94. [Crossref] [PubMed]
- Joshi VD, Kalvakolanu DV, Cross AS. Simultaneous activation of apoptosis and inflammation in pathogenesis of septic shock: a hypothesis. FEBS Lett 2003;555:180-4. [Crossref] [PubMed]
- Horne BD, Anderson JL, John JM, et al. Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol 2005;45:1638-43. [Crossref] [PubMed]
- Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest 2003;111:1805-12. [Crossref] [PubMed]
- Wyllie DH, Bowler IC, Peto TE. Bacteraemia prediction in emergency medical admissions: role of C reactive protein. J Clin Pathol 2005;58:352-6. [Crossref] [PubMed]
- Fock RA, Blatt SL, Beutler B, et al. Study of lymphocyte subpopulations in bone marrow in a model of protein-energy malnutrition. Nutrition 2010;26:1021-8. [Crossref] [PubMed]
- Vicente N, Cardoso L, Barros L, et al. Antithyroid drug-induced agranulocytosis: state of the art on diagnosis and management. Drugs R D 2017;17:91. [Crossref] [PubMed]


