Silencing or not silencing p63 in cardiac fibroblast, risks and benefits
Most heart diseases are often accompanied by a severe loss of cardiomyocytes (CMs) and by a pathological remodeling of the heart, culminating in heart failure or even sudden death (1). The very limited regenerative capacity of CMs is the main problem for heart repair. Cardiac reprogramming has emerged as a promising approach for cardiac regenerative therapy. Several strategies have been developed in order to get new CMs, including induced pluripotent stem cells (iPSCs) and its differentiation into CMs (2,3); activation of cardiac stem cells or stimulation of CMs to re-enter the cell cycle (4); and direct reprogramming or transdifferentiation of cardiac fibroblasts (CFs) to CMs (5,6).
CFs are fully differentiated mesenchymal cells and they play a key role in extracellular matrix (ECM) homeostasis in the heart. In response to a profibrotic stimulus, CFs predominantly proliferate and synthesize ECM and growth factors, leading to the formation of fibrosis and myocardial remodeling (7). Moreover, they have been termed as sentinel cells due to its capacity to respond to an inflammatory stimulus and also to secrete cytokines and chemokines enhancing cardiac inflammation response (8). CFs has been identified as an ideal cell source for direct reprogramming into CMs (9). After myocardial infarction, the fibroblasts expand and constitute the majority of the cells in the infarct zone (10,11). Therefore, reprogramming CFs into CMs represents a promising and beneficial approach and will be revolutionary for heart regeneration.
In 2010, was reported that mouse cardiac and dermal fibroblasts could be directly reprogrammed into induced CM-like cells (iCMs) in-vitro by a combination of 3 developmental cardiac transcription factors, Gata4, Mef2c, and Tbx5 (5). Several reviews highlight the potential and challenges of this new avenue for cardiac regenerative medicine (6). However, direct CFs reprogramming is accomplished through the activation of cardiac gene expression but must also be accompanied by complete silencing of the original CFs signature.
p63 belongs to the p53 family of transcription factors, together with p53 and p73. They are involved in development, differentiation and cell response to stress. However, in the absence of stress, the most important role of the p53 family is to regulate differentiation and development. It is crucial for this activity that p53 family members are expressed as two groups of proteins with opposing activity. N-truncated isoforms of p63 (DNp63) and p73 (DNp73) support self-renewal of the stem cells, while full-length isoforms of p53, p63 (TAp63), and p73 (TAp73) induce differentiation (12). Therefore, the balance of various p53 family isoforms may determine cell fate. Recent studies have demonstrated novel functions for TAp63 that could have potential implications to human pathologies. The first discovery is related to the protective role of TAp63 on premature aging. TAp63 controls skin homeostasis by maintaining dermal and epidermal progenitor/stem cell pool and protecting them from senescence, DNA damage and genomic instability, whereas the second study is related to the role of TAp63, expressed by the primitive endoderm, on heart development (13).
In an interesting and novelty approach, Patel et al., demonstrated that downregulation of p63 may activate cell reprogramming into CMs-like cells. In human CFs, adult murine CFs, and murine embryonic fibroblasts p63 downregulation showed CMs-like cells phenotype (14). Certainly, these outstanding results open new promissory possibilities and strategies to increase CMs-like cells in damaged and infarcted cardiac tissue. Cardiac-specific marker troponin T (cTnT) was increased in p63-downregulated CFs, even more after treatment with Hand2-Myocardin. Moreover, in co-culture with neonatal rat CMs, in these p63-knockout adult murine CFs close to CMs were discovered transient changes in calcium ion concentration and electrically-stimulated contractions, suggesting electric coupling. Finally, p63 downregulation did not increase cell proliferation, meaning that cell tumor growth effect was absent. Undoubtedly, this work enhances the use of growth factors cocktails to reprogram CFs in CMs; and even more, with the silencing or downregulation of p63, the percentage of reprogrammed cells is certainly higher even in human cells (14). This new strategy to directly reprogram local cells to damaged tissue in more differentiated cells opens new perspectives for the reprogramming of different tissues in different organs. Thus, the work of Patel et al., opens up flourishing perspectives in the field of tissue regeneration and not just cardiac regeneration.
All these results should be taken with caution before doing translational medicine. Obviously, there is still much work to be done regarding the reprogramming of fibroblasts based on the p63 downregulation. Perhaps the closest and immediate need is to know what could be the consequences of p63 silencing for CFs regarding secretion of growth factors, cytokines, chemokines and collagen homeostasis; besides proliferation and especially their differentiation to cardiac myofibroblasts (CMFs). All these elements and processes are necessary to repair damaged cardiac tissue after an injury; however, in this matter, the role of p63 in CFs remain unknown (Figure 1).
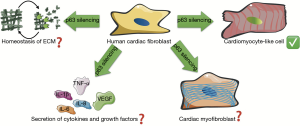
Respect fibroblast proliferation, different expression levels of DNp63 isoforms have been found in fibroblast from normal skin, keloid, and hypertrophic scars. In fact, the levels of nuclear DNp63 are greater in human keloid fibroblast than in human hypertrophic scar fibroblast, whereas p53 has minor levels in keloid fibroblast. This unbalances between DNp63 and p53 with the ability of DNp63 to block p53 increases even more the oncogenic role of N-truncated isoform. In fact, DNp63 upregulation and p53 downregulation could explain the aberrant growth of keloid scars (15). In this regard, it is important to note that in the work of Patel et al., the downregulation of p63 in FC did not show oncogenic activity; however, this imbalance between DNp63 and p53 could reduce the proliferative capacity of the CFs and thus affect the healing process.
On the other hand, there are no reports of how the silencing of p63 in CFs can affect their differentiation to CMFs. These cells are highly secretory of ECM proteins, and they play an important role in the closure of wounds due to their contractile property, and therefore, also play a key role in the process of tissue healing. However, in in-vitro and in-vivo studies miR-125b was identified as a novel regulator of cardiac fibrosis and proliferation, necessary and sufficient for the induction of fibroblast-to-myofibroblast transition. Furthermore, was showed that miR-125b inhibits p53 to induce fibroblast proliferation (16). Thus, the balance between DNp63 and p53 could also be important for CFs to CMFs differentiation.
With regard to the secretion of ECM proteins, mainly collagen, to date there are no antecedents that relate p63 to the synthesis and/or secretion of collagen in CFs. Secretion of growth factors is necessary for cardiac repair and is an important signature of CFs. To date, in mouse embryonic fibroblast the over-expression of DNp63 and TAp63 has been shown, exerts opposite effects on the levels of vascular endothelial growth factor (17); this mediator is key in tissue repair process due to its role in neoangiogenesis.
Finally, it is important to ask what could be the impact of p63 silencing in CFs and CMFs population due to the direct reprogramming of CFs into CMs-like cells. Therefore, without an in-vivo model to evaluate the loss of CFs and eventually CMFs in the heart, the research of this topic should consider the impact on the loss of these cell types in the repair process. Although many challenges and obstacles remain in this growing research field, the high demand for regenerative medicine strategies for the heart emphasizes the significance of these efforts in discovering new therapeutic strategies. Undoubtedly the findings of Patel et al., are the initial step to further determine in-vivo, what would be the functional benefits of the silencing of p63 in CFs oriented towards reprogramming into CMs. We should to be endeavoring to translate direct cardiac reprogramming for future clinical applications.
Acknowledgements
Funding: This work was supported by FONDECYT grant 1170425 to G. Díaz-Araya; and FONDAP ACCD is grant 15130011.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Harvey PA, Leinwand LA. The cell biology of disease: cellular mechanisms of cardiomyopathy. J Cell Biol 2011;194:355-65. [Crossref] [PubMed]
- Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007;131:861-72. [Crossref] [PubMed]
- Kattman SJ, Witty AD, Gagliardi M, et al. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell 2011;8:228-40. [Crossref] [PubMed]
- Tane S, Kubota M, Okayama H, et al. Repression of cyclin D1 expression is necessary for the maintenance of cell cycle exit in adult mammalian cardiomyocytes. J Biol Chem 2014;289:18033-44. [Crossref] [PubMed]
- Ieda M, Fu JD, Delgado-Olguin P, et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 2010;142:375-86. [Crossref] [PubMed]
- Fu JD, Srivastava D. Direct reprogramming of fibroblasts into cardiomyocytes for cardiac regenerative medicine. Circ J 2015;79:245-54. [Crossref] [PubMed]
- Souders CA, Bowers SL, Baudino TA. Cardiac fibroblast: the renaissance cell. Circ Res 2009;105:1164-76. [Crossref] [PubMed]
- Díaz-Araya G, Vivar R, Humeres C, et al. Cardiac fibroblasts as sentinel cells in cardiac tissue: Receptors, signaling pathways and cellular functions. Pharmacol Res 2015;101:30-40. [Crossref] [PubMed]
- Danilova N, Sakamoto KM, Lin S. p53 family in development. Mech Dev 2008;125:919-31. [Crossref] [PubMed]
- Brown RD, Ambler SK, Mitchell MD, et al. The cardiac fibroblast: therapeutic target in myocardial remodeling and failure. Annu Rev Pharmacol Toxicol 2005;45:657-87. [Crossref] [PubMed]
- Shinde AV, Frangogiannis NG. Fibroblasts in myocardial infarction: a role in inflammation and repair. J Mol Cell Cardiol 2014;70:74-82. [Crossref] [PubMed]
- Bourdon JC. p53 Family isoforms. Curr Pharm Biotechnol 2007;8:332-6. [Crossref] [PubMed]
- Paris M, Rouleau M, Pucéat M, et al. Regulation of skin aging and heart development by TAp63. Cell Death Differ 2012;19:186-93. [Crossref] [PubMed]
- Patel V, Singh VP, Pinnamaneni JP, et al. p63 Silencing induces reprogramming of cardiac fibroblasts into cardiomyocyte-like cells. J Thorac Cardiovasc Surg 2018;156:556-65.e1. [Crossref] [PubMed]
- De Felice B, Ciarmiello LF, Mondola P, et al. Differential p63 and p53 expression in human keloid fibroblasts and hypertrophic scar fibroblasts. DNA Cell Biol 2007;26:541-7. [Crossref] [PubMed]
- Nagpal V, Rai R, Place AT, et al. MiR-125b Is Critical for Fibroblast-to-Myofibroblast Transition and Cardiac Fibrosis. Circulation 2016;133:291-301. [Crossref] [PubMed]
- Senoo M, Matsumura Y, Habu S. TAp63gamma (p51A) and dNp63alpha (p73L), two major isoforms of the p63 gene, exert opposite effects on the vascular endothelial growth factor (VEGF) gene expression. Oncogene 2002;21:2455-65. [Crossref] [PubMed]


