Suturing of the laser resection area is recommended over a depth of 2 cm in an experimental porcine lung model
Introduction
Many solid tumors develop lung metastases during the course of the disease (1). If there is no other distant metastasis or tumor involvement of the mediastinal lymph nodes and if the actual tumor disease appears controlled, surgical removal may be useful (1,2). Lung metastases, if technically and anatomically possible, are removed non-anatomically. For this purpose, many thoracic surgeons use an Nd:YAG laser (wavelength 1,320 nm) (3,4). Either a focusing handheld device or, more recently, laser fibers are employed. Due to their small diameters laser fibers are particularly suitable for video thoracoscopic surgery. Lasers produce a high-energy light beam that impinges directly onto the lung tissue. The central tissue affected by the beam is vaporized due to very high temperatures. Towards the periphery, the energy and consequently the tissue temperature decrease. Depending on the applied laser energy, a coagulation layer of varying thickness will be formed. This coagulation layer homogeneously covers the entire resection surface, which in turn facilitates surgical procedures without blood loss. Using this laser technology, one or more lung metastases can be removed (5). Even if tumors are located centrally, a complete removal is possible. Depending on the depth of parenchymal defects, bronchopleural fistulae may occur, which may persist and necessitate a reoperation. Some experts therefore recommend closure of all resection surfaces by additional sutures (1,4,6). Unfortunately, this implicates a risk of restriction as a result of suturing on lung parenchyma. If many lesions are treated in this manner, the affected lung will ultimately not sufficiently expand. Furthermore, a residual thoracic cavity carrying the risk of infection may ensue. Suturing of the lung parenchyma prolongs (7) the surgical procedure and can be technically complex, in particular with video-thoracoscopic access. It is therefore desirable to generally dispense with suture closures of resection surfaces. In a preliminary publication (8) we were able to show that superficial resections have no relevant leakage. However, as the depth of the resection increases, small segment bronchi are injured. These are usually transected by the laser beam and tend to cause considerable air-loss. Local closure of these bronchi and suturing of the lung parenchyma becomes necessary.
Evidence regarding critical resection depth in laser surgery of the lung is scarce, reliable data and surgical guidelines have as yet not been presented.
In this experimental approach we now investigated the resection depth after non-anatomical laser resection of lung parenchyma in with regard to the resulting air leakage and the influence of additional suturing of the lung parenchyma.
Methods
From freshly slaughtered pigs weighing 90 kg each (average approximate weight), the heart and both lungs were removed en bloc. The trachea was severed below the larynx. All preparations were inspected for external integrity. Preparations with injuries of the lungs were discarded. Immediate transport to our laboratory followed. Preparations were unpacked and reassessed for integrity. The trachea was intubated via a ventilation tube (Vygon 520 Ch: 8.0, Braun Melsungen, Germany). The preparation was fixed on a board (see Figure 1) and placed into a waterproof container. The tube was connected to a respirator (Cicero EM, PM 8060, Dräger Lübeck, Germany). The peripheral areas of the lungs, where laser resections were intended were surface-marked with a stamper (circle with a diameter of 1.5 cm). Cylindrical lesions with a predefined and protocolled depth were created using an 800 µm laser fiber with an Nd:YAG LIMAX® 120 laser (Gebrüder Martin & Co. KG, Tuttlingen, Germany). The following resection depths were investigated applying laser energies of 40 and of 60 watts: 0.5, 1, 1.5 and 2.0 cm. Each resection was repeated 12 times. The container was then filled with water. To test for air leaks normal frequent (10/min) ventilation with an inspiratory peak pressure of 25 mbar and a PEEP of 5 mbar ensued. This defined pressure is equal to the pressure employed intraoperatively in a clinical setting (7,9). To quantitatively measure air leakage, a grid-shaped insert was placed on the lung surface so that the resection surfaces remained uncovered by the insert. The lung was gently submerged under water (see Figure 2). Air loss across the resection area was assessed according to the following established score (6-8). Score 0 = resection surface airtight, score 1 = leakage of isolated air bubbles, score 2 = exit of more than 5 bubbles per respiration and score 3 = massive air leakage. In order to quantify the air leakage more precisely, a water-filled volumetric flask with a funnel was pushed over the lasered lesion. Leaking air was collected through the funnel as it rose to the top of the flask. The quantity of air thus collected was read from the volumetric flask. Ventilation was stopped and the resection surfaces were coagulated for 5 seconds with a laser power of 60 watts at a distance of 1 cm (approximately) from the laser fiber to the lung surface. Testing for air leakage was repeated as previously described.
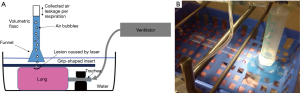
After completion of this second testing cycle, respiration was discontinued and the original resection surface was closed with a suture (PDS 2, USP 4-0, Johnson & Johnson Medical GmbH, Norderstedt, Germany). A third testing regarding airtightness was carried out in the same manner as before.
Individual groups were compared by the nonparametric paired two-tailed Mann-Whitney test. Significance was P<0.05. The Graph Prism software Version 6.0 (La Jolla, CA, USA) was used for statistical evaluation.
To determine thickness of the coagulation layer in lung parenchyma, resection sites were taken for histological examination. The sections were stained with hematoxylin-eosin (HE). Thickness of the coagulation layer was determined histologically employing the software Image J Version 1.51d (National Institutes of Health, Bethesda, USA). Averages and standard deviations were calculated.
Results
Airtightness of resection surfaces at laser power of 40 watts
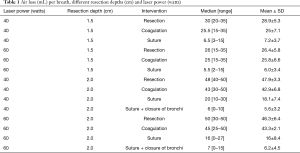
Up to a resection depth of 1.5 cm, all coagulated surfaces were completely airtight. Additional coagulation of the resection area for 5 seconds did not change this result. At a resection depth of 1.5 cm, air loss occurred from all resection surfaces at an average score of 2. For the ventilation parameters defined above, a leakage volume of 28.9±5.3 mL/ventilation was measured. After additional coagulation the average score was still 2 and the air volume was 25.0±7.1 mL/ventilation. There was no significant difference between coagulation and no coagulation (P=0.16). However, additional suturing was able to significantly reduce air losses to an average score of 1 and a leakage volume of 7.2±3.7 mL/ventilation (P<0.0001, Table 1, Figure 3).
Full table
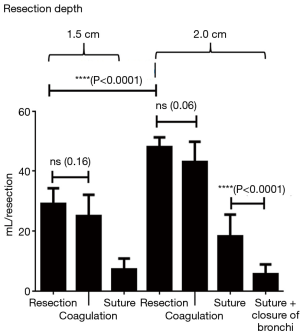
At a resection depth of 2 cm an average score of 3 was found and air leakage increased significantly to 47.9±3.3 mL/respiration. The difference compared to a depth of 1.5 cm was significant (P<0.0001). Upon inspection of the resection surfaces, individual opened segmental bronchi were identified. Additional coagulation of the resection surface did not significantly reduce the air loss in these specimen (P=0.06). Only suturing resulted in a highly significant (P<0.0001) reduction in air loss to an average score of 1 and a leak volume of 18.1±7.4 mL/ventilation. Even further reduction in air loss was achieved with suture closure of the segmental bronchi, followed by over-suturing of the resection surfaces. The resulting leakage volume was 5.6±3.2 (see Table 1 and Figure 3).
Histological sections showed an intact homogeneous coagulation layer, the underlying lung tissue was unaltered. The thickness of the coagulation layer was 63.3±0.8 µm before and after additional coagulation 63.3±1.0 µm (P=0.9).
Airtightness of the resection surfaces at a laser power of 60 watts
Up to a resection depth of 1.5 cm, all resection surfaces were completely airtight. These surfaces were homogeneously coagulated when viewed and individual bronchi were not visible. Relevant air losses occurred starting from a depth of 1.5 cm. Individual opened bronchi were not visible though. The mean score of leakage was 2 and leakage volume amounted to 26.4±5.8 mL/ventilation. After additional coagulation leakage volume did not decrease significantly (P=0.83) to 25.8±6.6 mL/respiration. Additional suturing, however, reduced the leakage volume significantly (P<0.0001) to a leakage volume of 6.0±3.4 mL/ventilation (see Table 1 and Figure 4).
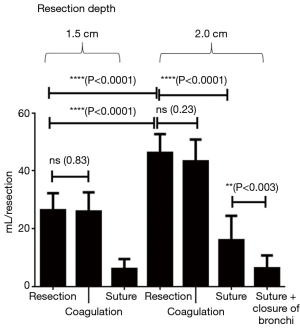
After laser resection reaching a depth of 2 cm showed homogeneously coagulated tissue, but also the lumina of very small opened bronchi. As a result, the score for all preparations was 3 and air leakage volume was 46.3±2.1 mL/ventilation. The difference compared to the resection depth of 1.5 cm was highly significant (P<0.0001). Additional coagulation revealed a non-significant (P=0.23) slight decrease in air leakage to 43.3±2.1 mL/ventilation. However, over-suturing significantly reduced air loss (P<0.0001) to a volume of 16.0±8.4 mL/ventilation. If the opened bronchi were selectively closed by sutures followed by a second over-and-over suture of the lung parenchyma, air loss could be lowered further to 6.2±4.5 mL/respiration (see Table 1 and Figure 4).
Histological examination of the resection surfaces (HE staining) showed a homogeneous coagulation zone with a thickness of 97.3±1.5 µm. The underlying lung parenchyma was unchanged. This thickness was almost the same at 97.9±1.5 µm after additional coagulation. Again, in this regard, there was no significant difference between coagulation and non-coagulation (P=0.36).
Comparison of airtightness as a function of the laser powers 40 and 60 watts
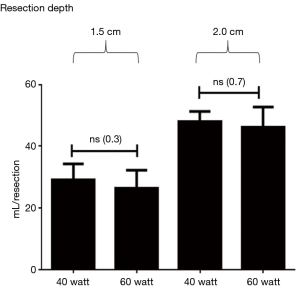
At a resection depth of 1.5 cm, there was no significant difference between resections with a laser power of 40 watts and those with 60 watts in terms of airtightness (P=0.3). The same applies to the resection depths of 2.0 cm (P=0.7) (see Figure 5).
In contrast, histological examination of the thickness of coagulation layers showed a highly significant difference of 63.2±0.86 µm (40 watts) vs. 97.3±1.5 µm (60 watts) (P<0.0001).
Discussion
Laser application to the lung parenchyma results in a coagulated surface of varying thickness depending on laser power. This coagulation is not only hemostatic, but seals small air leakages. Nevertheless, after ventilation of the lungs, air losses can occur to varying degrees. Our own preliminary work (8,9) indicated that the extent of these air losses depends on resection depth. Since minimally invasive surgery is becoming increasingly important in clinical practice (10,11), we performed our investigations with a laser fiber (800 µm), as is mainly used for such procedures. In order to produce reproducible lesions with a defined depth, we opted for an ex vivo model of pork lungs. The model was developed specifically for the investigations presented and the aim was to define properties for examination of parenchymal airtightness close to clinical conditions in the operating theatre.
It was found that up to a resection depth of 1.5 cm, both, a laser power of 40 watts and at 60 watts, the resection surfaces were completely airtight. This confirmed the results of our preliminary investigation (8). In this publication we investigated so called “peripheral resections”, which—based on the current results—we are now able to define more accurately. This indicates, that the resection area up to a depth of 1.5 cm may not require any additional procedure to remain airtight.
From a depth of 1.5 cm onwards, measurable air losses occurred. The mean in the 40-watt group was 28.9±5.3 mL compared to 26.4±5.8 mL in the 60-watt group. Both loss rates did not differ significantly (P=0.3) from each other. Visual inspection of the resection area showed homogenous coagulated tissue. Interestingly, the second step of additional coagulation of the resection surface did not significantly reduce air losses. The thought that further coagulation would produce a thicker coagulation layer and thus provides better sealing of the surface was mistaken. This may be due to our specific parameters of laser coagulation. Only an over-and-over suture of the resection surface led to significant reduction of air losses. In case of doubt this seems to be an effective safety precaution. At a resection depth of 2 cm, the lumina of smaller segmented bronchi became visible at the base of the resections. Laser powers of 60 watts, but also of 40 watts are obviously sufficient for their transsection. A significant increase of air leakage resulted in the experiment. By slicing unperforated fresh pork lungs with a sharp knife at a distance of 2 cm from the surface, the same observation was made (see Figure 6). At this resection depth, additional coagulation (60 watts) of the resection surface failed as a means of sealing the area. No significant reduction of the air loss was achieved in any of the specimen, segmental bronchi remained open on inspection (see Figure 7). Suturing of the resection surfaces reduced air losses by approximately 50% of the initial measurements. However, they remained considerably high. Only specific suturing of individual segment bronchi combined with a superficial over-and-over suture provided acceptable air sealing.
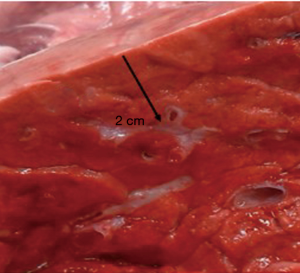
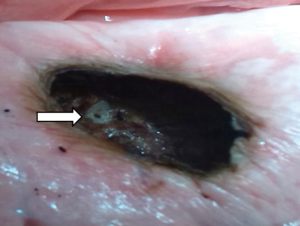
Due to anatomical lung structure with increasing depth opening of segmental bronchi was no surprise. The question was, whether laser application would be able to reliably close these bronchi. In our experiment this was clearly the case up to resection depths of maximum 1.5 cm, but not, if surgical strategy required deeper incisions.
Similar to the intraoperative clinical setting we obtained only results at a very early time interval after resection. We were not able to observe air losses over longer periods of time, during which an increase is possible. The clinical significance and its therapeutic consequences of accurately determining volume of air loss is currently unclear. Air losses from superficial lesions with a score of 2 usually close spontaneously with time and surgical reintervention is rarely needed (12-14).
It is of course difficult to draw conclusions for clinical practice. Human lungs may react differently from pig lungs. In vivo tissue per se will perhaps lead to different results. Nevertheless, our study adds some evidence to the growing field of minimally invasive laser resections. As a next step, in vivo experiments could help to further expand data finally leading to clinical studies.
Conclusions
In a model of non-anatomical ex vivo porcine lung, laser resection air loss via resection surfaces depend on resection depth. Starting at a depth of 1.5 cm, a closing suture of the resection is needed to avoid air leakage.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Osei-Agyemang T, Ploenes T, Passlick B. Pulmonary metastasectomy: indication and technique. Zentralbl Chir 2012;137:234-41. [PubMed]
- Pastorino U, Buyse M, Friedel G, et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg 1997;113:37-49. [Crossref] [PubMed]
- Rolle A, Kozlowski M. Laser resection of lung parenchyma--a new technical and clinical approach. Rocz Akad Med Bialymst 2005;50:193-6. [PubMed]
- Venuta F, Rolle A, Anile M, et al. Techniques used in lung metastasectomy. J Thorac Oncol 2010;5:S145-50. [Crossref] [PubMed]
- Baier B, Kern A, Kaderali L, et al. Retrospective survival analysis of 237 consecutive patients with multiple pulmonary metastases from advanced renal cell carcinoma exclusively resected by a 1318-nm laser. Interact Cardiovasc Thorac Surg 2015;21:211-7. [Crossref] [PubMed]
- Rolle A, Pereszlenyi A, Koch R, et al. Laser resection technique and results of multiple lung metastasectomies using a new 1,318 nm Nd:YAG laser system. Lasers Surg Med 2006;38:26-32. [Crossref] [PubMed]
- Macchiarini P, Wain J, Almy S, et al. Experimental and clinical evaluation of a new synthetic, absorbable sealant to reduce air leaks in thoracic operations. J Thorac Cardiovasc Surg 1999;117:751-8. [Crossref] [PubMed]
- Kirschbaum A, Steinfeldt T, Gockel A, et al. Airtightness of lung parenchyma without a closing suture after atypical resection using the Nd:YAG Laser LIMAX 120. Interact Cardiovasc Thorac Surg 2014;18:92-5. [Crossref] [PubMed]
- Kirschbaum A, Hochsmann N, Steinfeldt T, et al. Investigations of initial airtightness after non-anatomic resection of lung parenchyma using a thulium-doped laser with different optical fibres. Lasers Med Sci 2016;31:1097-103. [Crossref] [PubMed]
- Meyer C, Bartsch D, Mirow N, et al. Video-Assisted Laser Resection of Lung Metastases-Feasibility of a New Surgical Technique. Thorac Cardiovasc Surg 2017;65:382-6. [Crossref] [PubMed]
- Ng CSH, Capili F, Zhao ZR, et al. Laser resection of pulmonary nodule via uniportal thoracoscopic surgery. J Thorac Dis 2017;9:846-8. [Crossref] [PubMed]
- Kim WH, Lee HC, Ryu HG, et al. Intraoperative ventilatory leak predicts prolonged air leak after lung resection: A retrospective observational study. PLoS One 2017;12. [Crossref] [PubMed]
- Zaraca F, Vaccarili M, Zaccagna G, et al. Can a standardised Ventilation Mechanical Test for quantitative intraoperative air leak grading reduce the length of hospital stay after video-assisted thoracoscopic surgery lobectomy? J Vis Surg 2017;3:179. [Crossref] [PubMed]
- Isaka T, Kanzaki M, Onuki T. Technique for combined application of fibrin sealant and bioabsorbable felt against alveolar air leakage. Eur J Cardiothorac Surg 2008;34:705-author reply 706. [Crossref] [PubMed]