Expression and pathological significance of CC chemokine receptor 7 and its ligands in the airway of asthmatic rats exposed to cigarette smoke
Introduction
Asthma incidence is strongly related to air pollution levels, and cigarette smoke is among the most common indoor air pollutants. Cigarette smoke exposure alters immune response allergens in the lungs and is regarded as a major risk factor for asthma pathogenesis and exacerbation (1-3). A hyperactive inflammatory response in lung tissue is a defining characteristic of asthma. Recent studies have shown that lymphocytes and dendritic cells (DCs) play important roles in the development of asthma. Chemokine receptor 7 (CCR7) is involved in the process of DCs and T cell migration to lymphoid tissues and subsequent induction of the immune response (4-6). However, few studies have analyzed the direct effect of cigarette smoke on CCR7 expression, the T helper cell (Th)1/Th2 balance, and the immune inflammatory response in asthma.
This study was aimed to shed light on the relationship between lung CCR7 expression, Th1/Th2 balance and immune response in asthma rat models with or without cigarette smoke exposure.
Methods
Animals
Male Wistar rats (n=40) were provided by the Shanxi Medical University Center of Laboratory Animals, and were housed in specific-pathogen-free (SPF) environment. All experimental procedures were approved by Animal Care Committee of Shanxi Medical University, and completed in compliance with Chinese Council of Animal Care Guidelines. These rats were randomized to four groups (n=10 each): the control group, asthma group, smoke exposure group, and asthma with smoke exposure group.
Modeling of asthma in rats
Asthma was modeled in rats as previously described (7). Briefly, the rats in the asthma groups (with and without subsequent smoke exposure, n=20) were sensitized by two intraperitoneal injections (100 µg per dose) of ovalbumin (OVA, Sigma, St. Louis, MO, USA) adsorbed in 400 µg alum and dissolved in 0.2 mL normal saline (sensitization phase). Injections were separated by 1 week. Rats were then exposed to an aerosol containing 1% OVA for 30 minutes per day, 6 days each week, for up to 8 weeks (challenge phase). The rats in the smoke exposure groups (with and without prior asthma induction, n=20) were exposed to the smoke of ten unfiltered cigarettes (of a commercial product) for 1 hour per day, 6 days each week, for up to 8 weeks in a 91×59×62 cm3 box using a previous method (8). All rats in the control group were sensitized and challenged with sterile normal saline.
Western blotting of CCR7
Lung tissues were homogenized in radio immunoprecipitation assay (RIPA) lysis buffer and the protein levels were determined with bicinchoninic acid (BCA) protein assay kit (Thermofisher Scientific, Rockford, USA). The total protein was separated on 10% SDS-PAGE gels at 30 µg per lane and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, USA). Membranes were blocked in 5% non-fat dry milk, incubated with a 1:1,000 primary anti-rat CCR7 antibody (Abcam, Cambridge, USA) overnight at 4 °C, and then with a corresponding secondary anti-mouse or anti-rabbit IgG peroxidase (1:5,000) (Santa Cruz Biotechnology, Dallas, USA) at room temperature for 45 min. Anti-β-actin was employed as the gel loading control (1:1,000, Santa Cruz Biotechnology, Dallas, USA). The bands were visualized by autoradiography and quantified by densitometry. The measurements were normalized to β-actin. All experiments were repeated three times.
Immunohistochemistry of CCR7 and OX62
For immunohistochemical staining, paraffin embedded lung tissue samples were treated with xylene and ethyl alcohol, followed by incubation in 0.3% methanol/H2O2 to block endogenous peroxidases. Antigen retrieval was performed by boiling the mounted sections in 10 mM citrate (pH 6.0) solution for 20 min. The sections were then incubated overnight at 4 °C with primary antibodies. The two-step technique was used for visualization, with 3,3’-diaminobenzidine (DAB) and 0.02% H2O2 as chromogens. The sections were counterstained with hematoxylin. Primary antibodies for immunohistochemistry included anti-rat CCR7 (Santa Cruz) and anti-rat OX62 (Santa Cruz).
Cytokine measurement of peripheral blood and BALF
After serum and BLAF supernatant were collected, enzyme-linked immunosorbent assays (ELISAs) were used for measuring the levels of IL-4, INF-γ, CCL19, and CCL21 according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN, USA).
Statistical analysis
Statistical analysis was analyzed using SPSS 13.0 software (SPSS Inc., USA). The data are existed in form of mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was used to compare means among groups, followed by the SNK-q test for pair-wise comparisons. P values below 0.05 were considered statistically significant.
Results
Modeling of asthma in rats
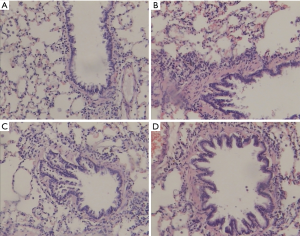
All rats in the asthma group and the asthma with smoke exposure group exhibited irregular respiratory rhythm, limb tremor, cough, and poor activity. Retarded reaction and pale color hair were occasionally noted. These manifestations were not seen in the control group. In addition, hematoxylin and eosin (H&E)-staining revealed inflammatory infiltration in the peribronchiolar area of the asthma, smoke exposure, and asthma with smoke exposure groups. Moreover, the thickness of bronchial wall or the smooth muscle layer was higher in the asthma, smoke exposure, and asthma with smoke exposure groups compared with the control group (Figure 1). In addition, we also measured the cell classification of alveolar lavage fluid. The results showed that the number of neutrophils in the asthma group was higher than that in the control group. The total number of white blood cells and neutrophils in the asthma with smoke exposure group was increased compared with the asthma group.
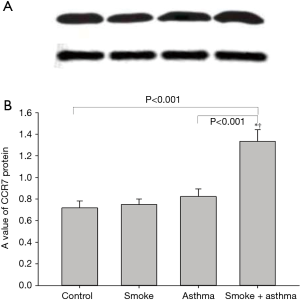
Effect of cigarette smoke exposure on CCR7 protein in the airway
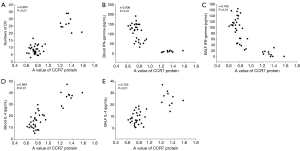
Both the smoke exposure and asthma groups exhibited slightly higher levels of CCR7 protein in lung tissue than the control group, while the asthma with smoke exposure group exhibited significantly elevated lung tissue CCR7 expression compared with all other groups (Figures 2,3).
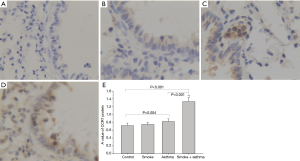
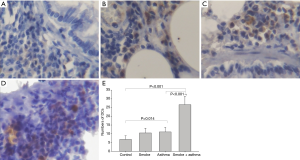
Effect of cigarette smoke exposure on number of DCs in the airway
OX-62 is a relatively specific antigen expressed by rat DCs, and was used as a marker to count the DCs population in airway tissue. The DCs number did not differ between the control and smoke exposure groups, while the asthma group showed more DCs than did the control group. Moreover, the number of DCs was higher in the asthma with smoke exposure group compared with the control group (Figure 4).
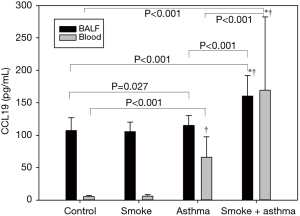
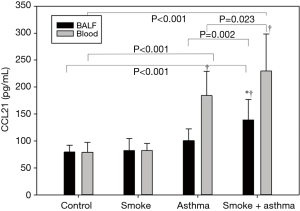
Effect of cigarette smoke exposure on CCL19 and CCL21 protein in peripheral blood and BALF
There were no differences in CCL19 and CCL21 protein expression levels in BALF among control, smoke exposure, and asthma groups, but these levels were significantly higher in the asthma with smoke exposure group compared with the control and asthma groups. There were no differences in CCL19 and CCL21 protein expression levels in peripheral blood between control and smoke exposure groups, while expression levels were significantly higher in asthma and asthma with smoke exposure compared with the control group (Figures 5,6).
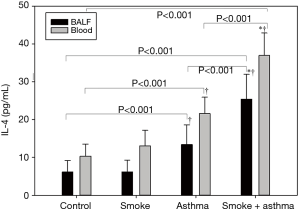
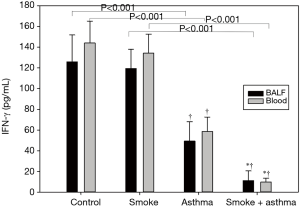
Effect of cigarette smoke exposure on INF-γ and IL-4 protein in the peripheral blood and BALF
We also found changes in cytokine concentrations related to Th1/Th2 balance in both peripheral blood and BALF. INF-γ was lower and IL-4 higher in both peripheral blood and BALF of the asthma group compared with the control group. In addition, INF-γ expression was lower and IL-4 significantly higher in the asthma with smoke exposure group compared with the asthma group (Figures 7,8).
Correlation analysis
CCR7 expression in airway tissue was positively correlated with the number of DCs as well as with IL-4 expression in peripheral blood and BALF, and negatively correlated with INF-γ in peripheral blood and BALF (Figure 9).
Discussion
Chemokines are a group of cytokines with similar structures and chemotactic functions. They promote the migration of inflammatory cells such as lymphocytes, monocytes, neutrophils, and DCs by interacting with corresponding chemokine receptors, and play important roles in both immune function and inflammatory mechanisms of allergic diseases (9). CCR7 is a 7-transmembrane domain, G-protein-coupled receptor mainly localized on the surfaces of dendritic, initial B, T, and Treg cells that induces asymmetric intracellular signaling pathways for directional migration. CCL19 and CCL21 are the major chemokine ligands of CCR7. In addition, CCL21 is expressed by high endothelial venules (HEVs) in lymph nodes and by lymphatic endothelial cells in most non-lymphoid tissues (10). There is no substantial difference in CCR7 affinity between CCL19 and CCL21, but they induce distinct intracellular signals (11). While CCL19/CCR7 signaling may be involved in the proliferation of smooth muscles in the airway (12), CCL21/CCR7 signaling is a more potent directional cue for DCs and lymphocyte migration than CCL19/CCR7 signaling (13). In the present study, we the expression levels of CCR7 and its ligands were shown to be increased in asthmatic rats after exposure to smoke, suggesting that smoke exposure exacerbates immune cell infiltration in asthma.
Many clinical studies found smoking has deleterious effects on lung function, such as a rapid reduction in forced expiratory volume in one second and reduced peak expiratory flow as well as lowered responsivity to routine asthma treatment (14,15). Moreover, asthmatic smokers are also more likely to develop severe asthma (16,17). It was known that asthma is a heterogeneous airway disorder with different phenotypes (18-20) among which asthma with smoke exposure is considered a special one (21). The purpose of our designed experiment is to further explain the clinical features of asthmatic smokers.
DCs are the predominant antigen-presenting cells in the mucosa of the respiratory tract (22). After infection and early inflammation, immature DCs infiltrate inflammatory sites through chemotaxis, where they identify and transform antigens into proteolytic peptides, and present them to lymphocytes. Thus, DCs build up an efficient defense network in the airway. In this process, immature DCs gradually become mature, and their function changes significantly with upregulated expression of the costimulatory molecules CD80, CD86, and CCR7 (23). Recent studies also have showed that DCs trigger activation and proliferation of T-lymphocytes through costimulatory molecules and MHC-II expressed on cell surface; in addition, they initiate T-cell polarization and differentiation signals via secretion of soluble cell factors such as prostaglandin E2 and IL-6, IL-12 (24). All of these factors play important roles in the pathogenesis of asthma.
Cigarette smoke exposure can damage airway structure through various processes and affect airway immune function. It was demonstrated that airway Th2 cell function, neutrophil infiltration, inflammatory cytokine levels (such as that of tumor necrosis factor, TNF) are enhanced by smoke exposure (25,26). In the present study, we observed that DCs were increased around the airway in asthmatic rats and that this response was amplified after exposure to smoke. DCs may be involved in the inflammatory response of airways in asthma after exposure to smoke. Moreover, we found the number of DCs around the airway positively correlated with CCR7 expression, so we speculate that cigarette smoke exposure can upregulate the number of DCs and then generate a range of immunoreactions through CCR7-mediated chemotaxis.
INF-γ is secreted by Th1 cells, as is IL-4 by Th2 cells, exclusively. These cytokines may modify expansion of other cell types, thereby regulating the Th1/Th2 balance (27,28). INF-γ can effectively activate mononuclear macrophages, enhance macrophage cytotoxic capacity, and reduce the synthesis of IgE (29). IL-4 can induce B cell proliferation, and stimulate differentiation of B cells towards IgE-generating plasmacytes, and promote native T cell differentiation towards Th2 cells involved in the inflammation response in allergic asthma (30). In the present research, we revealed abnormal secretion of both INF-γ and IL-4 in peripheral blood and BALF of asthmatic rats, which was further aggravated with exposure to smoking. Therefore, we speculated that cigarette smoke may interfere with the airway immune balance and worsen the airway inflammation in asthmatics. We observed that CCR7 expression was negatively correlated with INF-γ expression and positively correlated with IL-4 expression, suggesting that the effects of smoke on Th1/Th2 balance may be partly induced by CCR7. Further study is required to explore the precise mechanisms.
In conclusion, cigarette smoke exacerbated CCR7 expression, increased the number of DCs in airway tissue, and altered both systemic and airway expression levels of IL-4 and INF-γ in asthmatic rats. In addition, smoking enhance blood and BALF levels of the CCR7 ligands CCL19 and CCL21. Collectively, these results indicate that exposure to cigarette smoke affects the immune balance and immune response in asthma. All these changes may aggravate airway immunopathology. Hopefully further study on the signaling pathways induced by CCR7 activation in asthma with cigarette smoke exposure will show a new direction for treatment of these patients.
Acknowledgements
Funding: This study was sponsored by the National Natural Science Foundation of China (No. 81573245;81102198); the grants from Shanxi Scholarship Council of China (No. 2014-focus 8); Shanxi provincial health and Family Planning Commission projects (No. 2014169).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: All experimental procedures were approved by Animal Care Committee of Shanxi Medical University (No.2014-kp-8) and completed in compliance with Chinese Council of Animal Care Guidelines.
References
- Gautier C, Charpin D. Environmental triggers and avoidance in the management of asthma. J Asthma Allergy 2017;10:47-56. [Crossref] [PubMed]
- Mazenq J, Dubus JC, Gaudart J, et al. Air pollution and children's asthma-related emergency hospital visits in southeastern France. Eur J Pediatr 2017;176:705-11. [Crossref] [PubMed]
- Schultz ES, Litonjua AA, Melen E. Effects of long-Term exposure to traffic-related air pollution on lung function in children. Curr Allergy Asthma Rep 2017;17:41. [Crossref] [PubMed]
- El-Gammal A, Oliveria JP, Howie K, et al. Allergen-induced changes in bone marrow and airway dendritic cells in subjects with asthma. Am J Respir Crit Care Med 2016;194:169-77. [Crossref] [PubMed]
- Leόn B. T cells in allergic asthma: key players beyond the Th2 pathway. Curr Allergy Asthma Rep 2017;17:43. [Crossref] [PubMed]
- Mackenzie B, Andrade-Sousa AS, Oliveira-Junior MC, et al. Dendritic cells are involved in the effects of exercise in a model of asthma. Med Sci Sports Exerc 2016;48:1459-67. [Crossref] [PubMed]
- Dandurand RJ, Wang CG, Laberge S, et al. In vitro allergic bronchoconstriction in the brown Norway rat. Am J Respir Crit Care Med 1994;149:1499-505. [Crossref] [PubMed]
- Xu S, Wu R, Chen C. The study of E-cadherin expression on injury and repair of epithelium of respiratory tract in smoking mice. Zhonghua Jie He He Hu Xi Za Zhi 1999;22:417-9. [PubMed]
- Palomino DC, Marti LC. Chemokines and immunity. Einstein 2015;13:469-73. [Crossref] [PubMed]
- Cyster JG. Chemokines and cell migration in secondary lymphoid organs. Science 1999;286:2098-102. [Crossref] [PubMed]
- Comerford I, Harata-Lee Y, Bunting MD, et al. A myriad of functions and complex regulation of the CCR7/CCL19/CCL21 chemokine axis in the adaptive immune system. Cytokine Growth Factor Rev 2013;24:269-83. [Crossref] [PubMed]
- Kaur D, Saunders R, Berger P, et al. Airway smooth muscle and mast cell-derived CC chemokine ligand 19 mediate airway smooth muscle migration in asthma. Am J Respir Crit Care Med 2006;174:1179-88. [Crossref] [PubMed]
- Nandagopal S, Wu D, Lin F. Combinatorial guidance by CCR7 ligands for T lymphocytes migration in co-existing chemokine fields. PloS One 2011;6. [Crossref] [PubMed]
- Hayes CE, Nuss HJ, Tseng TS, et al. Use of asthma control indicators in measuring inhaled corticosteroid effectiveness in asthmatic smokers: a systematic review. J Asthma 2015;52:996-1005. [Crossref] [PubMed]
- Jerzynska J, Stelmach I, Grzelewski T, et al. High exposure to passive tobacco smoking and the development of asthma in an adult patient who had never smoked. Am J Respir Crit Care Med 2010;182:433-4. [Crossref] [PubMed]
- Dick S, Doust E, Cowie H, et al. Associations between environmental exposures and asthma control and exacerbations in young children: a systematic review. BMJ Open 2014;4. [Crossref] [PubMed]
- Hollenbach JP, Schifano ED, Hammel C, et al. Exposure to secondhand smoke and asthma severity among children in Connecticut. PloS One 2017;12. [Crossref] [PubMed]
- Cowan DC, Cowan JO, Palmay R, et al. Effects of steroid therapy on inflammatory cell subtypes in asthma. Thorax 2010;65:384-90. [Crossref] [PubMed]
- Gibson PG. Inflammatory phenotypes in adult asthma: clinical applications. Clin Respir J 2009;3:198-206. [Crossref] [PubMed]
- Jones AC, Bosco A. Using Network Analysis to Understand Severe Asthma Phenotypes. Am J Respir Crit Care Med 2017;195:1409-11. [Crossref] [PubMed]
- Hekking PP, Bel EH. Developing and emerging clinical asthma phenotypes. J Allergy Clin Immunol Pract 2014;2:671-80. [Crossref] [PubMed]
- Lambrecht BN, Hammad H. The role of dendritic and epithelial cells as master regulators of allergic airway inflammation. Lancet 2010;376:835-43. [Crossref] [PubMed]
- Johnson LA, Jackson DG. Control of dendritic cell trafficking in lymphatics by chemokines. Angiogenesis 2014;17:335-45. [Crossref] [PubMed]
- Audiger C, Rahman MJ, Yun TJ, et al. The Importance of Dendritic Cells in Maintaining Immune Tolerance. J Immunol 2017;198:2223-31. [Crossref] [PubMed]
- Nouri-Shirazi M, Guinet E. A possible mechanism linking cigarette smoke to higher incidence of respiratory infection and asthma. Immunol Lett 2006;103:167-76. [Crossref] [PubMed]
- Van Hove CL, Moerloose K, Maes T, et al. Cigarette smoke enhances Th-2 driven airway inflammation and delays inhalational tolerance. Respir Res 2008;9:42. [Crossref] [PubMed]
- Saini C, Tarique M, Rai R, et al. T helper cells in leprosy: An update. Immunol Lett 2017;184:61-6. [Crossref] [PubMed]
- Zhang Y, Zhang Y, Gu W, et al. TH1/TH2 cell differentiation and molecular signals. Adv Exp Med Biol 2014;841:15-44. [Crossref] [PubMed]
- Kamogawa Y, Minasi LA, Carding SR, et al. The relationship of IL-4- and IFN gamma-producing T cells studied by lineage ablation of IL-4-producing cells. Cell 1993;75:985-95. [Crossref] [PubMed]
- Yang F, Wang D, Li Y, et al. Th1/Th2 Balance and Th17/Treg-Mediated Immunity in relation to Murine Resistance to Dextran Sulfate-Induced Colitis. J Immunol Res 2017;2017. [Crossref] [PubMed]


