Characteristics and survival difference of clinical tumor size 0 extensive-stage small cell lung cancer with different metastasis pattern
Introduction
Small cell lung cancer (SCLC) is a highly aggressive malignancy frequently presenting with metastases at time of diagnosis (1-3). The standard treatment for extensive stage disease SCLC (ES-SCLC) is chemotherapy alone (4-6). Although 70% response rates to chemotherapy is found in ES-SCLC, the 5-year survival rates are still poor and only about 2% (7). Despite changes in demographics and treatment, the median and 5-year survival rates for patients with SCLC have not significantly improved over the past 15 years (8,9). To explore and understand the individualized characteristics of ES-SCLC may have some guiding significance for future treatment. Interestingly, small number of ES-SCLC patients who have no primary lesions [diagnosed as clinical tumor size 0 (cT0) according to American Joint Committee on Cancer (AJCC)] but have distant metastasis are found in clinical practice. What are the clinical characteristics and survival prognosis for these patients? However, few studies have been focused on the interesting clinical issue. In our study, we give a clear answer based on a large population screened from Surveillance, Epidemiology, and End Results (SEER) registered database.
Methods
Patient selection
SEER is supported by the Surveillance Research Program, which provides national leadership in the science of cancer surveillance as well as analytical tools and methodological expertise in collecting, analyzing, interpreting, and disseminating reliable population-based statistics (10). Included patients should be microscopically-confirmed and have only one primary tumor; diagnosed as the cT0 stage according to 7# AJCC staging extracted from the SEER database, and the cT0 was defined as having no primary tumor (11); diagnosed as ES-SCLC with distant metastasis. Importantly, all included patients should have definite cancer specific survival (CSS) and over survival (OS) information. CSS is a net survival measure representing survival of a specified cause of death in the absence of other causes of death. In addition, the variables including age, race, sex, N stage, metastasis sites should have clear information. For the radiation information, we can only know if the patients have received radiotherapy. However, the we cannot know the specific radiotherapy site due to the limitations of SEER database itself.
Ethical evaluation
As previous described (12), this study was conducted in compliance with the Helsinki Declaration. The ethics of this study has been approved by Shandong Cancer Hospital. The patient’s informed consent is not required due to the SEER database does not involve personal identifying information.
Statistical analysis
The suitable patients are screened by the SEER*Stat 8.3.4 software from 2010 to 2013. The patient’s baseline characteristics were compared by chi-square test. The CSS and OS were regarded as the main study endpoint. The survival curve was depicted by the Kaplan-Meier method and the statistic difference was compared by the Log rank test. The related risk factors on cT0 stage were compared by the Univariate and multivariate Logistic regression analysis. The influence of T stage on CSS and OS was analyzed by the univariate and multivariate Cox regression model.
The SPSS version 22.0 (SPSS, IL, Chicago, USA) was applied to compare the statistical difference. P values were 2-sided and the P<0.05 was considered statistically significant.
Results
Patient demographics
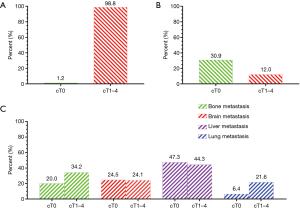
In total of 9,046 patients were included in our study, and only 110 patients (1.2%) were diagnosed at cT0 stage according to the AJCC staging (Figure 1A). Among them, 90% of patients were Caucasian. The statistic differences were not found in subgroup variables including age, sex and radiation between the patients with cT0 stage and cT1–4 stage (all, P>0.05). Interestingly, 30.9% of patients at cT0 stage were diagnosed at the clinical nodal status 0 (cN0) stage, however, only 12.0% of patients at cT1–4 stage was diagnosed at cN0 stage (Figure 1B). In addition, our results showed that the patients with cT0 stage have fewer bone metastasis and lung metastasis (bone metastasis: 20% for cT0 stage, 34.2% for cT1–4 stage, P=0.002; lung metastasis: 6.4% for cT0 stage, 21.6% for cT1–4 stage, P<0.001) (Figure 1C). Statistic differences between the two groups were not found in patients with brain and liver metastasis (P=0.906 for brain metastasis; P=0.540 for liver metastasis) (Figure 1C). The detailed statistical information was presented in Table 1.
Full table
Factors associated with cT0 stage
Our results demonstrated that the variables including age, race, sex and radiation were not risk factors on cT0 stage. Interestingly, the cN0 stage was showed to be an independent risk factor on cT0 stage [N1 vs. N0: odds ratio (OR): 0.291; 95% confidence interval (CI): 0.113–0.749, P=0.010; N2 vs. N0: OR:0.290; 95% CI: 0.184–0.457, P<0.001; N3 vs. N0: OR:0.456; 95% CI: 0.273–0.762, P=0.003] (Table 2). Importantly, the patients with bone and lung metastasis were less likely to belong to cT0 stage (bone metastasis: OR:0.521; 95% CI: 0.325–0.835, P=0.007; lung metastasis: OR:0.257; 95% CI: 0.119–0.553, P=0.001) (Table 2). However, the brain and liver metastasis were proven not to be risk factors on cT0 stage (P=0.993 for brain metastasis; P=0.827 for liver metastasis) (Table 2). Interestingly, the radiation information was also proven not to be a risk factor on cT0 stage (P=0.205) (Table 2).
Full table
Survival difference for patients with cT0 stage
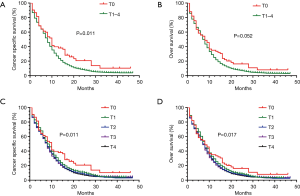
The results demonstrated that the patients with cT0 stage had better CSS compared with the cT1–4 stage (P=0.011) (Figure 2A). However, OS difference was not found between the patients with cT0 stage and the cT1–4 stage (P=0.052) (Figure 2B). Next, we divided T stage into cT0, cT1, cT2, cT3, cT4, and CSS and OS benefit could be found in patients with cT0 stage (P=0.011 for CSS; P=0.017 for OS) (Figure 2C,D).
Next, we adjusted for age, race, sex, stage N, stage T, bone metastasis, brain metastasis, liver metastasis, lung metastasis and radiation, and multivariate Cox regression model was used to analyze the prognostic factors for OS and CSS. The results showed that the cT0 stage was an independent prognostic factor for CSS [hazard ratio (HR): 1.318; 95% CI: 1.049–1.656; P=0.018] (Table 3). However, the cT0 stage was not an independent prognostic factor for OS (HR: 1.220; 95% CI: 0.985–1.511; P=0.068) (Table 3).

Full table
Discussion
In our present study, we mainly focused on the characteristics and survival prognostic of ES-SCLC patients with cT0 stage. Several interesting clinical issues were clarified for the first time. Firstly, we found the patients at cT0 stage only accounts for 1.2% of these included patients. Secondly, we found the patients at cT0 stage are less likely to develop lymph nodes metastasis. Thirdly, the patients at cT0 stage had fewer bone and lung metastasis. Last but not the least, the patients at cT0 stage had better CSS benefit, however, an OS benefit was not found in our present study.
We demonstrated that the patients at cT0 stage was a different subgroup compared with the patients at cT1–4 stage for ES-SCLC patients with distant metastasis. Only 1.2% ES-SCLC patients with distant metastasis were diagnosed at cT0 stage in our study. In fact, tumor size is an important factor affecting metastasis of SCLC (13). However, for these patients who lack primary tumor, what is their metastasis pattern? Our results showed that these patients had fewer lung and bone metastasis. However, such a difference was not found in patients with liver and brain metastasis between the patients at cT0 stage and cT1–4 stage. In fact, brain, bone, liver and lung were the most common metastatic site for SCLC (14). Our previous study had demonstrated that the liver was the most common metastatic site, and lung was the least common metastatic site for these patients (15). Interestingly, we also found that 47.3% of patients developed liver metastasis and only 6.4% of study subjects have lung metastasis at cT0 stage in SEER database.
Our results also demonstrated that improved CSS could be found in patients at cT0 stage. Several reasons should be clarified: Firstly, the patients at cT0 stage had less bone and lung metastasis. It has been demonstrated that multiple metastatic sites at diagnosis significantly predicted poor survival in ES-SCLC patients (2). Secondly, studies had showed that large tumor size at diagnosis tended to result in poor survival for these patients (2,16). Therefore, it is easy to understand that the patients at cT0 stage have better survival prognosis without primary lesions. Thirdly, the patients at cT0 stage were more likely to be at cN0 stage. Some studies had showed that the presence of thoracic lymph node involvement significantly affected the long-term survival (17,18). Interestingly, we found OS difference was not found between the patients with cT0 stage and the cT1–4 stage. Complications or treatment-related deaths from SCLC itself may be the cause of no difference. In our study, the results showed that the cT0 stage was an independent prognostic factor for CSS. In fact, a previous study has demonstrated that age less than 50 years, female sex, Asian race, and rural residence were associated with better CSS for SCLC (19). However, the patients in this study included localized SCLC and extensive SCLC. In our study, only the ES-SCLC patients with different metastasis were included.
Several study limitations need to be mentioned. Firstly, our study is a retrospective analysis of a large community-based data set and the selection bias cannot avoid despite of a relatively large sample size. Secondly, some important variables may predict for survival in these particular patients, including younger age, good performance status, nonsmoking history and the oligometastatic disease; however, they were not entered in this exploratory analysis (20,21). Thirdly, due to the limitations of the SEER database itself, we only analyzed the generic use of radiation excluding other treatments such as first line platinum-based chemotherapy, thoracic chemoradiotherapy, and recently involved second line immunotherapy. All these variables can influence outcomes and affect prognosis (7,22,23). In addition, the information which organ received radiation therapy was also lacked in our study duo to limitations of SEER database itself.
Conclusion, our results showed for the first time that ES-SCLC patients at cT0 stage had fewer bone and lung metastases and were more likely to be at cN0 stage. The CSS benefit could be found in patients with cT0 stage compared with cT1–4 stage. Our findings may provide some individualized insights and therapeutic perspectives for ES-SCLC patients with distant metastasis.
Acknowledgments
Funding: This study was supported jointly by the National Natural Science Foundation of China (No. 81603348); China Postdoctoral fund (No. 21300075311104) and Shandong postdoctoral innovation special fund (No. 201602012); China Postdoctoral Special Fund (No. 2018T110696); Shandong Province key R & D Plan (No. 2018GSF119014).
Footnote
Conflict of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The ethics of this study has been approved by Shandong Cancer Hospital. The patient’s informed consent is not required due to the SEER database does not involve personal identifying information.
References
- Schneider BJ, Kalemkerian GP. Personalized therapy of small cell lung cancer. Adv Exp Med Biol 2016;890:149-74. [Crossref] [PubMed]
- Fukui T, Itabashi M, Ishihara M, et al. Prognostic factors affecting the risk of thoracic progression in extensive-stage small cell lung cancer. BMC Cancer 2016;16:197. [Crossref] [PubMed]
- Rudin CM, Ismaila N, Hann CL, et al. Treatment of small-cell lung cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians Guideline. J Clin Oncol 2015;33:4106-11. [Crossref] [PubMed]
- Hanna N, Bunn PA Jr, Langer C, et al. Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol 2006;24:2038-43. [Crossref] [PubMed]
- Hermes A, Bergman B, Bremnes R, et al. Irinotecan plus carboplatin versus oral etoposide plus carboplatin in extensive small-cell lung cancer: A randomized phase III trial. J Clin Oncol 2008;26:4261-7. [Crossref] [PubMed]
- Kalemkerian GP, Akerley W, Bogner P, et al. Small cell lung cancer. J Natl Compr Canc Netw 2013;11:78-98. [Crossref] [PubMed]
- Pietanza MC, Byers LA, Minna JD, et al. Small cell lung cancer: will recent progress lead to improved outcomes? Clin Cancer Res 2015;21:2244-55. [Crossref] [PubMed]
- Gaspar LE, McNamara EJ, Gay EG, et al. Small-cell lung cancer: prognostic factors and changing treatment over 15 years. Clin Lung Cancer 2012;13:115-22. [Crossref] [PubMed]
- Wang S, Tang J, Sun T, et al. Survival changes in patients with small cell lung cancer and disparities between different sexes, socioeconomic statuses and ages. Sci Rep 2017;7:1339. [Crossref] [PubMed]
- Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database. Available online: https://seer.cancer.gov/data-software/documentation/seerstat/
- Vallières E, Shepherd FA, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals regarding the relevance of TNM in the pathologic staging of small cell lung cancer in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:1049-59. [Crossref] [PubMed]
- Lin J, Li C, Zhang C, et al. Postmastectomy radiation therapy for breast cancer patients with one to three positive lymph nodes: a propensity score matching analysis. Future Oncol 2017;13:1395-404. [Crossref] [PubMed]
- Tas F, Aydiner A, Topuz E, et al. Factors influencing the distribution of metastases and survival in extensive disease small cell lung cancer. Acta Oncol 1999;38:1011-5. [Crossref] [PubMed]
- Nakazawa K, Kurishima K, Tamura T, et al. Specific organ metastases and survival in small cell lung cancer. Oncol Lett 2012;4:617-20. [Crossref] [PubMed]
- Cai H, Wang H, Li Z, et al. The prognostic analysis of different metastatic patterns in extensive-stage small-cell lung cancer patients: a large population-based study. Future Oncol 2018;14:1397-407. [Crossref] [PubMed]
- Eerola AK, Soini Y, Pääkkö P. A high number of tumor-infiltrating lymphocytes are associated with a small tumor size, low tumor stage, and a favorable prognosis in operated small cell lung carcinoma. Clin Cancer Res 2000;6:1875-81. [PubMed]
- Billing PS, Miller DL, Allen MS, et al. Surgical treatment of primary lung cancer with synchronous brain metastases. J Thorac Cardiovasc Surg 2001;122:548-53. [Crossref] [PubMed]
- David EA, Clark JM, Cooke DT, et al. The Role of Thoracic Surgery in the Therapeutic Management of Metastatic Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:1636-45. [Crossref] [PubMed]
- Lara JD, Brunson A, Riess JW, et al. Clinical predictors of survival in young patients with small cell lung cancer: Results from the California Cancer Registry. Lung Cancer 2017;112:165-8. [Crossref] [PubMed]
- Hanagiri T, Takenaka M, Oka S, et al. Results of a surgical resection for patients with stage IV non-small-cell lung cancer. Clin Lung Cancer 2012;13:220-4. [Crossref] [PubMed]
- Liu T, Liu H, Wang G, et al. Survival of M1a Non-Small Cell Lung Cancer Treated Surgically: A Retrospective Single-Center Study. Thorac Cardiovasc Surg 2015;63:577-82. [Crossref] [PubMed]
- Ott PA, Elez E, Hiret S, et al. Pembrolizumab in Patients With Extensive-Stage Small-Cell Lung Cancer: Results From the Phase Ib KEYNOTE-028 Study. J Clin Oncol 2017;35:3823-9. [Crossref] [PubMed]
- Demedts IK, Vermaelen KY, Van Meerbeeck JP. Treatment of extensive-stage small cell lung carcinoma: current status and future prospects. Eur Respir J 2010;35:202-15. [Crossref] [PubMed]


