Pulmonary vasculitis
Introduction
Vasculitides are a heterogeneous group of diseases characterised by the inflammation and destruction of vessel walls, which reduces blood flow into tissues. These diseases are classified according to the affected organ and size of the vessels involved (1-4). Vasculitides can be primary or secondary to connective tissue disease, infection, neoplasm, or to a state of hypersensitivity (5). Primary vasculitides are rare diseases with an approximate incidence of 20–100 cases/million/year and a prevalence of 150–450 cases/million (5-7). Small-vessel vasculitides associated with antineutrophil cytoplasmic antibodies (ANCAs) generally affect the lungs.
Diagnosis is challenging and it is based on abnormal clinical, radiological, histopathological and analytical findings. More specifically, diagnosis is primarily based on ANCAs levels and other immunopathological markers (immunoglobulin A, cryoglobulin and anti-basement membrane antibodies). The primary objective of this article was to determine the features of primary systemic vasculitis with pulmonary involvement.
Normal pulmonary vessels
A pulmonary vessel is composed of three concentric layers called “tunics”: the inner layer (tunica intima), which contains endothelial cells and loose subendothelial connective tissue; the medial layer (tunica media), which is formed by concentric smooth muscle cells and variable amounts of elastin, reticular fibers and proteoglycans. It is more developed in arteries than in veins and is virtually absent from capillaries. The outer layer (tunica adventitia) is made up of collagen and elastic fibers. Its thickness is variable, being relatively thin in arteries and thick in venules and veins. It is the outer covering of blood vessels (vasa vasorum).
More specifically, pulmonary vessels contain a lower number of smooth muscle cells. Pulmonary vessels have a thin wall (a third the thickness of the aorta), which confers them more elasticity and enables their functioning at low pressure with high blood flows. There are two types of pulmonary arteries: muscle arteries—with a 100–1,000-µm diameter and a significantly thicker layer, and elastic arteries, which do not contain muscle cells and have a diameter of >1,000 µm (8).
Classification
The clinical and radiological features of vasculitides depend on the organ affected and size of the blood vessels involved, i.e., large-calibre (aorta and its main branches); medium-calibre (primary visceral arteries); and small-caliber vessels (arterioles, capillaries and venules). Therefore, clinical and radiological findings are major criteria for the classification of vasculitides, which is based on the Revised International Chapel Hill Consensus Conference Nomenclatures of Vasculitides of 2012 (Table 1) (9-11).

Full table
Pulmonary vasculitis
Vessel size is a useful clinical descriptor. However, the reason why a particular disease affects vessels of a specific diameter is unknown. Vessels of the same size are not necessarily identical, as they are shaped by the needs of their anatomical location during embryonic development. Thus, same-calibre vessels of distinct organs have different characteristics, since each vessel is designed to satisfy the local needs of the tissue it perfuses (12).
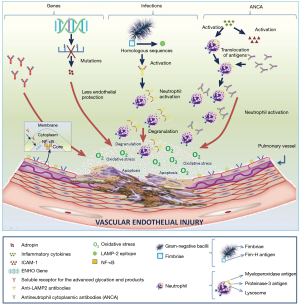
The etiology of these vasculitides is unknown, although interaction between genetic and environmental factors might play a role in the etiology of these diseases (12) (Figure 1).
Large-vessel vasculitis
Takayasu’s arteritis
Arteritis (which is often granulomatous) mainly affects the aorta and/or its branches of women <50 years old (9). Pulmonary arteritis is observed in 15–60% of patients (13). Initial symptoms usually are systemic and include malaise, low-grade fever and arthralgia (pre-ischemic phase). When the pulmonary artery is affected, it can cause dyspnea, coughing, chest pain, haemoptysis and, occasionally, pulmonary arterial hypertension. Late-term symptoms (ischemic phase) appear at an advanced stage and depend on the artery involved. These include variable pulse in the extremities and claudication of the vascular territory involved (10–25% of the cases). CT angiography and MRI scans can detect subtle changes in vessel walls, which helps differentiate current disease from stenosis caused by previously damaged vasculature. The use of positron emission tomography is, for the moment, the subject of intensive research (14). Diagnostic criteria are shown in Table 2 (15).

Full table
The treatment for pulmonary artery stenosis consists of high doses of corticosteroids occasionally combined with methotrexate and azathioprine. Cyclophosphamide has been traditionally used for refractory or severe disease, although it is being progressively replaced with new biological agents [tocilizumab (interleukin-6 receptor antagonist) and rituximab (monoclonal antibody anti-CD20, which reduces lymphocyte B count through various mechanisms)] (16). Therapeutical options for severe stenosis of the pulmonary artery include angioplasty, stent implantation, bypass and pulmonary artery (17,18).
Giant cell arteritis
Arteritis (which is often granulomatous) usually affects the aorta and/or its main arteries, with a predilection for the carotid branches, vertebral arteries and the temporal artery. It is observed in patients >50 years of age and is often associated with rheumatic polymyalgia (9). Histologically, it is indistinguishable from Takayasu’s arteritis and is 2–6 times more frequent in women than in men. Pulmonary involvement is rare (non-productive cough in 10% of patients) but it should be considered in patients of advanced age with recent pharyngeal pain, aphonia or cough of unknown origin. Findings on CT and MRI scans are similar to those in Takayasu’s arteritis. Diagnostic criteria are shown in Table 2 (19).
Treatment is based on corticosteroids (dose of 40–60 mg/day) (20) and results in rapid improvement. Methotrexate, cyclophosphamide and tocilizumab are therapeutic options in different settings (21). Like in Takayasu’s arteritis, some patients can benefit from surgical interventions.
Medium-vessel vasculitis
Polyarteritis nodosa
Polyarteritis nodosa is a necrotizing arteritis with segment involvement of medium- and small-calibre muscular arteries without glomerulonephritis or vasculitis in arterioles, venules and capillaries not associated with ANCAs (9). Up to 30% of patients present antigens against hepatitis B virus. Pulmonary involvement is rare and its presence indicates a disease other than polyarteritis nodosa (22).
Kawasaki disease
Kawasaki disease is an arteritis associated with mucocutaneous lymph-node syndrome and it predominantly affects medium and small arteries (9). Its most relevant complication is coronary artery damage, which ranges from transitory dilatation to vessel wall destruction, with the development of aneurysms (23). It generally occurs in children younger than 5 years (24). Pulmonary arteritis is observed in 45–71% of autopsies (25). The prevalence of pulmonary involvement is associated with ethnicity. In a case series study performed in Japan, 14.7% presented pulmonary alterations on chest X-ray (26). In contrast, in an Italian study in 250 patients, no pulmonary involvement was documented in any subject (27). Respiratory symptoms are secondary to diffuse interstitial lung disease (which generally manifests with a reticular/micronodular echo pattern) or to the presence of pulmonary infiltrates and pleural effusion (26).
Small-vessel vasculitis
This type of vasculitis can be categorized into ANCA-associated vasculitis (AAV) and immunocomplex vasculitis (Table 1). The difference lies in the absence or small number of immunocomplex vessel wall deposits found in AAV, which contrasts with the moderate to marked deposits observed in immunocomplex vasculitis.
ANCA-associated vasculitis
ANCAs
ANCAs are IgG-type antibodies against cytoplasmic antigens in the cytoplasm of neutrophils and monocytes [proteinase 3 (PR3) and myeloperoxidase (MPO)]—essentially azurophilic granules of polymorphonuclear neutrophils. ANCAs promote neutrophil migration and vessel wall degranulation, thereby triggering the release of proteases and other toxic metabolites responsible for vascular damage. In the context of endothelial cells, this process leads to endothelial detachment and lysis (28). The presence of ANCAs in more than 90% of microscopic polyangiitis (MPA) and granulomatosis polyangiitis (GPA) suggests a relevant role in the etiology of the disease. This hypothesis is supported by the correlation between ANCAs titre and AAV (ANCAs decrease when a treatment is administered and increase in relapse); the effectiveness of plasmapheresis and B-cell targeted therapies (i.e., rituximab), and by a case of maternal MPO-ANCAs transfer to the neonate associated with the development of nephritis and pulmonary hemorrhage in the neonate. Yet, ANCAs must not be the only etiological factor for ANCA-induced vasculitis. Thus, some patients with ANCAs-induced vasculitis phenotype are ANCAs-negative, whereas some patients in remission are ANCAs-positive (29). The most relevant ANCAs staining patterns characterized by indirect immunofluorescence are cytoplasmic (c-ANCAs; specific antibodies against PR3 that can be observed in GPA) and perinuclear [p-ANCAs; antibodies against MPO and other antigens in eosinophilic granulomatosis with polyangiitis (EGP) and MPA].
Genetic factors
Different genetic factors could influence the etiopathogenesis of AAV. Mutations in the energy homeostasis associated gene (gene ENHO) reduce adropin production (a protein that protects the vascular endothelium), which is associated with endothelial damage. There is evidence demonstrating that ENHO mutations and adropin deficiency play a relevant role in the activation of endothelial cells during neutrophil recruitment. ENHO mutations and adropin deficiency have also been documented to be involved in neutrophil-endothelial interaction in vascular inflammation induced by interleukin-1 and tumor necrosis factor-α (TNF-α). This status increases susceptibility to ANCAs-associated lung injury (30). AAV raises the levels of soluble receptors of advanced glycated end products (RAGE), which promotes the production of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-ĸβ) and initiates the inflammatory cascade involved in vascular damage (31).
Granulomatosis with polyangiitis (Wegener’s granulomatosis)
Granulomatous necrotizing inflammation affects the upper and lower airways, with necrotizing vasculitis mainly affecting medium and small vessels. It is common in pauci-immune necrotizing glomerulonephritis (9). The yearly incidence of this disease in Europe is 2.1–14.4 cases/million (32), and it occurs in all age groups.
Patients with GPA exhibit vascular cell adhesion molecule-1 (VCAM-1) overexpression in endothelial surface. VCAM-1 plays a crucial role in leukocyte recruitment and adhesion to the vascular endothelium, migration and extravasation. High levels of soluble VCAM-1 are a marker of endothelial cell activation in AAV (33). Soluble VCAM-1 levels are higher in GPA than in systemic sclerosis. High levels of soluble VCAM-1 are considered a marker of endothelial cell activation in AAV (33). Recent genetic studies have identified various loci strongly linked to predisposition to vasculitis, such as GPA and HLA-DP, PR3, SERPINA1 and semaphorin (SMA6A) (34).
Environmental factors—including exposure to toxic substances and infections—are known to be involved in the pathogenesis of vasculitis. Thus, two studies published in the 1990’s revealed that chronic nasal infection by Staphylococcus aureus may trigger GPA activity (35,36). Some years later, several studies showed that chronic nasal infection by Staphylococcus aureus is an independent risk factor for GPA relapse, being patients previously treated with trimethoprim-sulfamethoxazole less likely to experience relapse (37-39). It has been demonstrated that fimbriated-pathogen infection frequently precedes pauci-immune focal necrotizing glomerulonephritis (acute inflammatory disease that results in rapid, irreversible kidney failure, typically in the context of systemic small vessel vasculitis such as GPA or MPA). Fimbriae of gram-negative bacilli present anti-FimH antibodies that have homologous sequences with epitopes on lysosomal-associated membrane protein-2 (LAMP-2), which promotes the activation of anti-LAMP-2. These antibodies cause neutrophil activation, and subsequent degranulation causes vascular cell apoptosis, which suggests that infection in a susceptible host could trigger the production of antibodies (40). Thus, FimH-triggered autoimmunity to LAMP-2 is a clinically relevant molecular mechanism that could induce the development of pauci-immune focal necrotizing glomerulonephritis (Figure 1).
Between 25% and 55% of patients with GPA show lung involvement, which occurs more frequently in c-ANCAs positive patients (41). The classical clinic triad includes upper airway (sinusitis, otitis, ulcerations, subglottic and bronchial stenosis) and lower airway involvement (haemoptysis, thoracic pain, dyspnea and cough) and glomerulonephritis (haematuria, azotemia), although not all occur at presentation (only 40% of patients have renal involvement at that moment) (42). Patients who do not show signs of systemic vasculitis and exhibit specific pathological and clinical changes in the respiratory tract should be considered GPA patients (especially if they are ANCA-positive). The most frequent radiological findings include pulmonary nodules and masses in any location (90% of cases) (43) that can join masses >10 cm, cavitate and get infected (hydro-aerial level in the interior of the mass). A perinodular ground glass halo may be observed. Other findings include thickening of the bronchial walls, atelectasis, bronchiectasis or pleural effusion (44). ANCAs test is positive in >90% of cases (mainly c-ANCAs/anti-PR3 positive). Diagnostic criteria are shown in Table 2. Differential diagnosis with MPA is complex as granulomas are not observed in all samples.
The management of GPA and all AAVs is based on a range of recently published recommendations (45,46). Treatment consists of an initial “Remission induction therapy”, where a more intensive immunosuppressive therapy is administered to control the active illness, and a maintenance phase where the treatment is less intensive to minimize side effects while remission is maintained. When a new diagnosis of AAV—organ- or life-threatening—is confirmed, a combined treatment with glucocorticoids, cyclophosphamide or rituximab is recommended. If no organs are affected by AAV, management with a combination of methotrexate and mycophenolate is recommended. When AAV relapse occurs and compromises an organ or is life-threatening, the recommendation is the same as for newly diagnosed AAV (a combination of glucocorticoids and cyclophosphamide or rituximab). In patients with serum creatinine >5.7 mg/dL due to rapidly progressive glomerulonephritis—either de novo or recurrent—plasmapheresis should be considered. Plasmapheresis is also an option for the treatment of diffuse alveolar haemorrhage (DAH). In patients in remission, a combination of low doses of glucocorticoids and azathioprine, rituximab, methotrexate or mycophenolate for at least 24 months is recommended. For patients refractory to the treatment, it is recommended to replace cyclophosphamide with rituximab (or the other way round), refer the patient to an expert specialist, reconsider diagnosis, optimize the treatment, and arrange for inclusion in clinical trials. For decades, the standard treatment for patients with major organ involvement consisted of high doses of cyclophosphamide and glucocorticoids; consequently, approximately 75% of patients experienced remission within 3 months and 90% achieved it in 6 months. Yet, relapse and side effects were frequent (29). New recommendations are intended to limit exposure to cyclophosphamide and glucocorticoids during the induction and maintenance phase. Biological therapies (e.g., rituximab, among others) directed at cell and molecular components specific of autoimmune response and mediators of inflammatory damage could be more effective and less toxic (47). Patients treated with rituximab who exhibit a low proportion of B CD5+ cells relapse significantly earlier that the ones who have normal levels of B CD5+ cells. Thus, B CD5+ cells are considered an indicator of illness activity and guide remission maintenance therapy following treatment with rituximab (48).
MPA
MPA is a necrotizing vasculitis with little or no immune deposits (pauci-immune) that mainly affects small vessels. Recent genetic studies have identified various loci strongly associated with predisposition to vasculitis, most of which are important actors in immune and inflammatory response (49), as it occurs with MPA and HLA-DQ (50). Necrotizing glomerulonephritis is very common, pulmonary capillaritis is frequent and granulomatous inflammation is absent (9). Symptoms are discomfort, anorexia, fever, night sweats, arthromyalgia, weight loss and rapidly progressive glomerulonephritis (51). Pulmonary involvement is observed in 10–30% of cases usually in the form of DAH. Radiologically, a heterogeneous ground-glass pattern is observed accompanied by patchy and bilateral abnormalities suggesting DAH (52). ANCAs test is positive—mainly for p-ANCAs/anti-MPO—in 50–75% of patients. The treatment is the same as for AAV.
EGP
A granulomatous inflammation rich in eosinophils with necrotizing vasculitis mainly affecting medium and small vessels that is associated with asthma and eosinophilia. ANCAs test is positive in 45–70% of patients (mainly for p-ANCAs/anti-MPO). The presence of ANCAs is more frequent in the presence of glomerulonephritis (9). Moreover, genetic studies have identified a strong association between EGP and HLA-DRB4 that triggers an immune inflammatory response (53). Asthma and eosinophilia are relevant, and differential diagnosis should be done with allergic bronchopulmonary aspergillosis, cortico-dependent asthma and eosinophilic pneumonia, among others. There are two types of EGP: ANCAs-positive EGP—which affects the kidneys more frequently, and ANCAs-negative negative EGP—with more pronounced eosinophilia and severe pulmonary disease (54). Asthma is always present, is corticoid-dependent and, in the long term, usually precedes vasculitis. Rhinitis, nasal polyps or sinusitis are rarely ulcerating. Radiological images show ground-glass opacity; patchy, bilateral, homogeneous—occasionally migratory—infiltrates peripherally located; hyperinflation, bronchial wall thickening, and the characteristic mosaic pattern of severe asthma (55). A third of patients show pleural effusion, predominantly eosinophilic (56). Increased levels of eosinophils (>25%) in bronchoalveolar lavage contribute to the diagnosis. Transbronchial biopsy rarely provides a conclusive diagnosis. Diagnostic criteria are shown in Table 2. The treatment is the same as for AAV. Adding leukotrienes or omalizumab (monoclonal antibody anti-IgE) antagonists allows reducing the dose of corticosteroids in patients whose main limiting manifestation is asthma. This reduction of corticoids could help identify vasculitic manifestations of the illness (57,58).
Immune complex small vessel vasculitis
Anti-basement membrane antibodies disease
Anti-basement membrane antibodies disease is a type of vasculitis that affects glomerular/pulmonary capillaries—or both, and presents deposits of anti-glomerular basement antibodies. Pulmonary involvement causes pulmonary haemorrhage, renal glomerulonephritis and acute kidney failure (9). The term “Goodpasture disease” is used for patients who exhibit anti-glomerular basement antibodies, whereas Goodpasture syndrome is used to describe the coexistence of glomerulonephritis and DAH of any cause. The origin of this disease is unknown, although genetic and environmental factors seem to be involved (59). This disease is characterized by a bimodal presentation of age, its incidence peaking at thirty (prevalent in men with renal and pulmonary involvement) and at sixty/seventy (when isolated renal affectation is more frequent) (60). Pulmonary involvement (40–60% of patients, probably depending on the number of floating antibodies accessing the alveolar basement membrane) is less frequent than renal involvement, although the latter has also been documented (61). Radiological images are those characteristics of DAH. Lung biopsy shows alveolar haemorrhage with pulmonary capillaritis. Up to 20–30% of patients are also ANCAs-positive (generally against MPO) and usually show extra-renal involvement. Diagnosis is based on renal biopsy (lineal deposits of IgG in the glomerular membrane). The elective treatment is triple therapy [corticosteroids, immunosuppressors (generally cyclophosphamide) and plasmapheresis (to remove anti-glomerular basement antibodies)] (62). In dialysis patients, plasmapheresis is questionable since these patients do not recover renal function. Rituximab alone or associated with cyclophosphamide also provides good results (63).
Cryoglobulinemic vasculitis
Vasculitis with cryoglobulin immune deposits that affects the small vessels associated with seric cryoglobulins (9). Pulmonary involvement is rare (approximately 2% of patients). Radiological alterations are related to DAH (64), and prognosis is usually poor (65).
IgA vasculitis (Henoch-Schönlein purpura)
Small vessel vasculitis with IgA1-dominant immune deposits. This disease mainly affects children and usually involves the skin, peripheric nerves and glomeruli (glomerulonephritis indistinguishable from IgA nephropathy) (9). It is usually preceded by an upper airway infection, and infectious or chemical agents seem to be involved in its pathogenesis (66). Pulmonary involvement is rare in children and more frequent in adults. IgA deposits in the alveolar basement membrane lead to alveolitis. Radiological images show DAH (67) and pleural effusion (68).
Hypocomplementemic urticarial vasculitis
A type of vasculitis followed by urticaria and hypocomplementemia affecting the small vessels and associated with anti-C1q antibodies (69). It is often accompanied by glomerulonephritis, arthritis and ocular inflammation. Pulmonary affectation appears in 20% of patients, generally in the form of chronic obstructive pulmonary disease and bronchial asthma. Pulmonary vasculitis leads to the release of elastase by neutrophils and the development of panacinar pulmonary emphysema, mainly in basal zones (70). Pleural effusion has also been reported (71).
Various-calibre vessel diseases
Behçet disease
Vasculitis in patients with Behçet disease is characterised by recurring oral and/or genital aphthous ulcers and inflammatory lesions in the skin, eyes, joints, and in gastrointestinal and/or central nervous system. This type of vasculitis affects vessels of any size (20% arteries, 80% veins). Thromboangeitis, thrombosis, arteritis and arterial anaeurysm may also exist (9). It is generally observed in young men, with more cases in silk route countries, especially in Turkey (72). Thoracic involvement may be observed in less than 10% of cases (73), mainly in men. Clinical manifestations include haemoptysis, thoracic pain, cough and dyspnea (74). The most frequent findings are main and lobar pulmonary artery aneurysm—usually multiple and bilateral (75,76), superior caval vein obstruction, thromboembolism and pulmonary infarction (77). CT angiography may show affectation of the pulmonary artery (78-80). Peripheral vascular damage may also be present (superficial or deep vein thrombosis). The management of vein thrombosis is controversial. While some experts recommend immunosuppressive treatment, others support the combination of immunosuppressants with blood thinners (81). The treatment is a combination of high doses of corticosteroids with cyclophosphamide, azathioprine or infliximab (82-84). Anticoagulation is also used in patients with stenotic or occlusive disease. For refractory haemoptysis, surgical interventions such as endovascular embolization or lobectomy can be performed (85,86). Hughes-Stovin syndrome is a limited form of Behçet disease without oral or genital ulceration (87) with similar image findings (88).
Diagnosis
Medical records and physical examination
Vasculitides are difficult to diagnose, even for a well-trained specialist, as the symptoms of this rare group of diseases are similar to those of more common conditions (i.e., infection, neoplasm). However, there are particular signs that should raise suspicions, namely: (I) DAH [triad of diffuse alveolar infiltrates, hemoptysis (not always present) and dropped hematocrit followed by increased diffusion capacity by 30%]; (II) rapidly progressive glomerulonephritis [active urinary sediment (erythrocyte cylinders, haematuria with dysmorphic erythrocytes and proteinuria >500 mg/dL), elevated urea and creatinine serum levels, arterial hypertension and edema]; (III) lung-kidney syndrome (patients with DAH and glomerulonephritis); (IV) ulceration or deformity of upper airway lesions (for example, refractory chronic sinusitis with ulcers or soft tissue destruction); (V) cavitating lesions or pulmonary nodules on imaging; (VI) tangible purpura (suggests skin vasculitis); (VII) peripheral nervous system manifestations like mononeuritis; (VIII) Systemic disease (simultaneous presence of symptoms and signs that suggest simultaneous or sequential affectation of various organs). Cough, fever or dyspnea are frequent manifestations; yet, the most characteristic sign is the presence of DAH as a result of capillaritis and the subsequent destruction of the alveolar-capillary membrane. At the onset of DAH, patients may be asymptomatic (symptoms will appear over days) or show acute respiratory failure. The majority of patients experience haemoptysis, although approximately a third of patients won’t (89). Physical examination could reveal crepitant breathing.
Laboratory findings
The most frequent finding in DAH is anaemia, although leucocytosis may also appear. Lactate dehydrogenase values double the normal upper limit and are a risk factor for intrahospital mortality (90). Creatinine values may be elevated if DAH is a manifestation of a lung-kidney syndrome with concurrent glomerular disease. Elevated levels of eosinophils in peripheral blood and tissues in patients with EGP suggest that these cells play a role in the pathogenesis of the disease. CD95-induced inhibition of apoptosis prolongs the half-life of eosinophils, which decisively contributes to the development of chronic eosinophilia. Although the mechanism by which the activation of eosinophils causes endothelial damage is not completely understood, recent data suggest a potential role of T lymphocytes secreting eosinophil-activating cytokines (91).
Radiology
In DAH, high resolution thoracic computerised tomography (CT) may show bilateral consolidations, although diffuse bilateral ground glass areas can also be observed, sometimes restricted to the lower lobes (64). When clinical symptoms resolve, radiological images improve, although symptoms may persist some weeks after bleeding have been stopped. After DAH, a septal thickening called “beaded pattern” may be observed (92). Recurrent DAH can lead to pulmonary fibrosis. Thoracic CT scan will help discard other potential causes of pulmonary haemorrhage in patients with haemoptysis.
Bronchofibroscopy
In DAH, sequential aliquots of bronchoalveolar lavage look increasingly bloodier. Diagnosis of DAH is confirmed when at least 20% of the alveolar macrophages collected demonstrate hemosiderin deposits on iron staining. Yet, haemorrhage may not be detected if bronchoalveolar lavage is performed incorrectly.
Histopathology
The diagnostic profitability of the transbronchial biopsy is usually <10% (93). For small vessel diagnosis, a biopsy of the radiologically anomalous pulmonary parenchyma by videothoracoscopy is highly effective (94,95). Histopathological findings should be interpreted in conjunction with clinical and serological data, and final diagnosis should be confirmed by immunofluorescence. Comparative histopathological analysis of another tissue should also be performed, if available.
Figure 2 shows the algorithm with recommendations for the management of AAV.
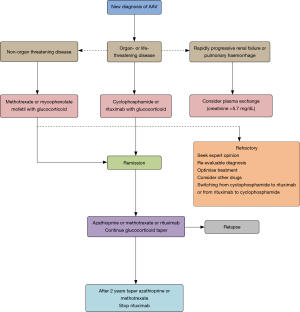
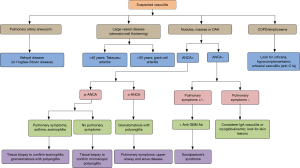
Figure 3 displays the diagnostic algorithm of pulmonary vasculitis that correlates radiological findings with clinical and laboratory findings (44).
To summarise, many systemic vasculitides may affect the pulmonary vasculature. Their signs and symptoms may be non-specific, and diagnosis becomes a real challenge. Laboratory findings contribute to reduce differential diagnosis, although overlapping with other vasculitides and pathological processes may occur. Imaging tests can reveal specific alterations that guide the clinician to the right diagnosis. Vascular pulmonary involvement is a severe manifestation of severe systemic vasculitis and generally requires immediate treatment with corticosteroids and immunosuppressants.
Acknowledgements
The authors would like to thank Fernando Vázquez-Vázquez for his help with the figures.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Frankel SK, Schawarz MI. The pulmonary Vasculitides. Concise clinical review. Am J Respir Crit Care Med 2012;186:216-24. [Crossref] [PubMed]
- Fauci AS, Haynes B, Katz P. The spectrum of vasculitis: clinical, pathologic, immunologic and therapeutic considerations. Ann Intern Med 1978;89:660-76. [Crossref] [PubMed]
- Elefante E, Tripoli A, Ferro F, et al. One year in review: systemic vasculitis. Clin Exp Rheumatol 2016;34:S1-6. [PubMed]
- Kallenberg CG. Pathogenesis and treatment of ANCA-associated vasculitides. Clin Exp Rheumatol 2015;33:S11-4. [PubMed]
- Brown KK. Pulmonary vasculitis. Proc Am Thorac Soc 2006;3:48-57. [Crossref] [PubMed]
- González-Gay MA, Garcia-Porrua C. Systemic vasculitis in adults in northwestern Spain, 1988-1997. Clinical and epidemiologic aspects. Medicine (Baltimore) 1999;78:292-308. [Crossref] [PubMed]
- Watts RA, Lane SE, Bentham G, et al. Epidemiology of systemic vasculitis: a ten-year study in the United Kingdom. Arthritis Rheum 2000;43:414-9. [Crossref] [PubMed]
- Peinado-Cabré VI, Pizarro-Serra S, Barberà-Mir JA. Circulación pulmonar. In: Casan-Clarà P, García-Río F, Gea-Guiral J. editors. Fisiología y biología respiratorias. Ergon edition. Majadahonda, Madrid, 2007;173-94.
- Jennette JC, Falk RJ, Bacon PA, et al. 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 2013;65:1-11. [Crossref] [PubMed]
- Gómez-Román JJ. Diffuse alveolar hemorrhage. Arch Bronconeumol 2008;44:428-36. [Crossref] [PubMed]
- Jennette JC, Falk RJ. Small-vessel vasculitis. N Engl J Med 1997;337:1512-23. [Crossref] [PubMed]
- Hoffman GS, Calabrese LH. Vasculitis: determinants of disease patterns. Nat Rev Rheumatol 2014;10:454-62. [Crossref] [PubMed]
- Manganelli P, Fietta P, Carotti M, et al. Respiratory system involvement in systemic vasculitides. Clin Exp Rheumatol 2006;24:S48-59. [PubMed]
- Arnaud L, Haroche J, Malek Z, et al. Is (18)F-fluorodeoxyglucose positron emission tomography scanning a reliable way to assess disease activity in Takayasu arteritis? Arthritis Rheum 2009;60:1193-200. [Crossref] [PubMed]
- Arend WP, Michel BA, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum 1990;33:1129-34. [Crossref] [PubMed]
- Clifford A, Hoffman GS. Recent advances in the medical management of Takayasu arteritis: an update on use of biologic therapies. Curr Opin Rheumatol 2014;26:7-15. [Crossref] [PubMed]
- Qin L, Hong-Liang Z, Zhi-Hong L, et al. Percutaneous transluminal angioplasty and stenting for pulmonary stenosis due to Takayasu’s arteritis: clinical outcome and four-year follow-up. Clin Cardiol 2009;32:639-43. [Crossref] [PubMed]
- Hamamoto M, Futagami D. Pulmonary artery replacement for pulmonary Takayasu’s arteritis. Gen Thorac Cardiovasc Surg 2012;60:435-9. [Crossref] [PubMed]
- Hunder GG, Bloch DA, Michel BA, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum 1990;33:1122-8. [Crossref] [PubMed]
- Dasgupta B, Borg FA, Hassan N, et al. BSR and BHPR guidelines for the management of giant cell arteritis. Rheumatology (Oxford) 2010;49:1594-7. [Crossref] [PubMed]
- Villiger PM, Adler S, Kuchen S, et al. Tocilizumab for induction and maintenance of remission in giant cell arteritis: a phase 2, randomized, double-blind, placebo-controlled trial. Lancet 2016;387:1921-7. [Crossref] [PubMed]
- Frankel SK, Sullivan EJ, Brown KK. Vasculitis: Wegener granulomatosis, Churg-Strauss syndrome, microscopic polyangiitis, polyarteritis nodosa, and Takayasu arteritis. Crit Care Clin 2002;18:855-79. [Crossref] [PubMed]
- Burns JC. The riddle of Kawasaki disease. N Engl J Med 2007;356:659-61. [Crossref] [PubMed]
- Jennette JC, Falk RJ. Nosology of primary vasculitis. Curr Opin Rheumatol 2007;19:10-6. [Crossref] [PubMed]
- Amano S, Hazama F, Kubagawa H, et al. General pathology of Kawasaki disease on the morphological alterations corresponding to the clinical manifestation. Acta Pathol Jpn 1980;30:681-94. [PubMed]
- Umezawa T, Saji T, Matsuo N, et al. Chest x-ray findings in the acute phase of Kawasaki disease. Pediatr Radiol 1989;20:48-51. [Crossref] [PubMed]
- Falcini F, Cimaz R, Calabri GB, et al. Kawasaki’s disease in northern Italy: a multicenter retrospective study of 250 patients. Clin Exp Rheumatol 2002;20:421-6. [PubMed]
- Kallenberg CG. Pathogenesis of ANCA-associated vasculitides. Ann Rheum Dis 2011;70:i59-63. [Crossref] [PubMed]
- Jennette JC, Nachman PH. ANCA Glomerulonephritis and Vasculitis. Clin J Am Soc Nephrol 2017;12:1680-91. [Crossref] [PubMed]
- Gao F, Fang J, Chen F, et al. Enho mutations causing low adropin: a possible pathomechanism of MPO-ANCA associated lung injury. EbioMedicine 2016;9:324-35. [Crossref] [PubMed]
- Kamo T, Tasaka S, Tokuda Y, et al. Levels of soluble receptor for advanced glycation end products in bronchoalveolar lavage fluid in patients with various inflammatory lung diseases. Clin Med Insights Circ Respir Pulm Med 2016;9:147-54. [PubMed]
- Gibelin A, Maldini C, Mahr A. Epidemiology and etiology of Wegener granulomatosis, microscopic polyangiitis, Churg-Strauss syndrome and Goodpasture syndrome: vasculitides with frequent lung involvement. Semin Respir Crit Care Med 2011;32:264-73. [Crossref] [PubMed]
- Kawano-Dourado L, Ab’Saber AM, Capelozzi VL, et al. In situ evidence of pulmonary endothelial activation in patients with granulomatosis with polyangiitis and systemic sclerosis. Lung 2015;193:355-9. [Crossref] [PubMed]
- Xie G, Roshandel D, Sherva R, et al. Association of granulomatosis with polyangiitis (Wegener’s) with HLA-DPB1*04 and SEMA6A gene variants: evidence from genome-wide analysis. Arthritis Rheum 2013;65:2457-68. [Crossref] [PubMed]
- Stegeman CA, Tervaert JW, Sluiter WJ, et al. Association of chronic nasal carriage of Staphyloccocus aureus and higher relapse rates in Wegener granulomatosis. Ann Intern Med 1994;120:12-7. [Crossref] [PubMed]
- Stegeman CA, Tervaert JW, de Jong PE, et al. Trimethoprim-sulfamethoxazole (co-trimoxazole) for the prevention of relapses of Wegener’s granulomatosis. Dutch Co-Trimoxazole Wegener Study Group. N Engl J Med 1996;335:16-20. [Crossref] [PubMed]
- Zycinska K, Wardyn KA, Zielonka TM, et al. Chronic crusting, nasal carriage of Staphylococcus aureus and relapse rate in pulmonary Wegener’s granulomatosis. J Physiol Pharmacol 2008;59:825-31. [PubMed]
- Laudien M, Gadola SD, Podschun R, et al. Nasal carriage of Staphylococcus aureus and endonasal activity in Wegener’s granulomatosis as compared to rheumatoid arthritis and chronic Rhinosinusitis with nasal polyps. Clin Exp Rheumatol 2010;28:51-5. [PubMed]
- Bonaci-Nikolic B, Andrejevic S, Pavlovic M, et al. Prolonged infections associated with antineutrophil cytoplasmic antibodies specific to proteinase 3 and myeloperoxidase: diagnostic and therapeutic challenge. Clin Rheumatol 2010;29:893-904. [Crossref] [PubMed]
- Kain R, Exner M, Brandes R, et al. Molecular mimicry in pauci-immune focal necrotizing glomerulonephritis. Nat Med 2008;14:1088-96. [Crossref] [PubMed]
- Hirayama K, Kobayashi M, Usui J, et al. Pulmonary involvements of antineutrophil cytoplasmic autoantibody-associated renal vasculitis in Japan. Nephrol Dial Transplant 2015;30:i83-93. [Crossref] [PubMed]
- Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med 1992;116:488-98. [Crossref] [PubMed]
- Lee KS, Kim TS, Fujimoto K, et al. Thoracic manifestation of Wegener’s granulomatosis: CT findings in 30 patients. Eur Radiol 2003;13:43-51. [PubMed]
- Mahmoud S, Ghosh S, Farver C, et al. Pulmonary vasculitis: Spectrum of imaging appearances. Radiol Clin N Am 2016;54:1097-118. [Crossref] [PubMed]
- Frankel SK, Jayne D. The pulmonary vasculitides. Clin Chest Med 2010;31:519-36. [Crossref] [PubMed]
- Yates M, Watts RA, Bajema IM, et al. EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann Rheum Dis 2016;75:1583-94. [Crossref] [PubMed]
- Specks U, Merkel PA, Seo P, et al. Efficay of remission-induction regimens for ANCA-associated vasculitis. N Engl J Med 2013;369:417-27. [Crossref] [PubMed]
- Bunch DO, McGregor JG, Khandoobhai NB, et al. Decreased CD51 B cells in active ANCA vasculitis and relapse after rituximab. Clin J Am Soc Nephrol 2013;8:382-91. [Crossref] [PubMed]
- Carmona FD, Martín J, González-Gay MA. Genetics vasculitis. Curr Opin Rheumatol 2015;27:10-7. [Crossref] [PubMed]
- Lyons PA, Rayner TF, Trivedi S, et al. Genetically distinct subsets whitin ANCA-associated vasculitis. N Engl J Med 2012;367:214-23. [Crossref] [PubMed]
- Gayraud M, Guillevin L, le Toumelin P, et al. Longterm followup of polyarteritis nodosa, microscopic polyangiitis, and Churg-Strauss syndrome: analysis of four prospective trials including 278 patients. Arthritis Rheum 2001;44:666. [Crossref] [PubMed]
- Primack SL, Miller RR, Müller NL. Diffuse pulmonary hemorrhage: clinical, pathologic, and imaging features. AJR Am J Roentgenol 1995;164:295-300. [Crossref] [PubMed]
- Vaglio A, Martorana D, Maggiore U, et al. Secondary and primary vasculitis study group. HLA-DRB4 as a genetic risk factor for Churg-Strauss syndrome. Arthritis Rheum 2007;56:3159-66. [Crossref] [PubMed]
- Sinico RA, Di Toma L, Maggiore U, et al. Prevalence and clinical significance of antineutrophil cytoplasmic antibodies in Churg-Strauss syndrome. Arthritis Rheum 2005;52:2926. [Crossref] [PubMed]
- Buschman DL, Waldron JA Jr, King TE Jr. Churg-Strauss pulmonary vasculitis. High-resolution computed tomography scanning and pathologic findings. Am Rev Respir Dis 1990;142:458-61. [Crossref] [PubMed]
- Lanham JG, Elkon KB, Pusey CD, et al. Systemic vasculitis with asthma and eosinophilia: a clinical approach to the Churg-Strauss syndrome. Medicine (Baltimore) 1984;63:65-81. [Crossref] [PubMed]
- Keogh KA, Specks U. Churg-Strauss syndrome: clinical presentation, antineutrophil cytoplasmic antibodies, and leukotriene receptor antagonists. Am J Med 2003;115:284-90. [Crossref] [PubMed]
- Wechsler ME, Wong DA, Miller MK, et al. Churg-Strauss syndrome in patients treated with omalizumab. Chest 2009;136:507. [Crossref] [PubMed]
- Hellmark T, Segelmark M. Diagnosis and classification of Goodpasture’s disease (anti-GBM). J Autoimmun 2014;48-49:108-12. [Crossref] [PubMed]
- McAdoo SP, Pusey CD. Anti-glomerular basement membrane disease. Clin J Am Soc Nephrol 2017;12:1162-72. [Crossref] [PubMed]
- Lombard CM, Colby TV, Elliott CG. Surgical pathology of the lung in anti-basement membrane antibody-associated Goodpasture’s syndrome. Hum Pathol 1989;20:445-51. [Crossref] [PubMed]
- Dammacco F, Battaglia S, Gesualdo L, et al. Goodpasture’s disease: a report of ten cases and a review of the literature. Autoimmun Rev 2013;12:1101-8. [Crossref] [PubMed]
- Syeda UA, Singer NG, Magrey M. Anti-glomerular basement membrane antibody disease treated with rituximab: a case-based review. Semin Arthritis Rheum 2013;42:567-72. [Crossref] [PubMed]
- Chung MP, Yi CA, Lee HY, et al. Imaging of pulmonary vasculitis. Radiology 2010;255:322-41. [Crossref] [PubMed]
- Ramos-Casals M, Robles A, Brito-Zerón P, et al. Life-threatening cryoglobulinemia: clinical and immunological characterization of 29 cases. Semin Arthritis Rheum 2006;36:189-96. [Crossref] [PubMed]
- Rigante D, Castellazzi L, Bosco A, et al. Is there a crossroad between infections, genetics, and Henoch-Schönlein purpura? Autoimmun Rev 2013;12:1016-21. [Crossref] [PubMed]
- Nadrous HF, Yu AC, Specks U, et al. Pulmonary involvement in Henoch-Schönlein purpura. Mayo Clin Proc 2004;79:1151-7. [PubMed]
- Cream JJ, Gumpel JM, Peachey RD. Schönlein-Henoch purpura in the adult. A study of 77 adults with anaphylactoid or Schönlein-Henoch purpura. Q J Med 1970;39:461-84. [PubMed]
- Wisnieski JJ, Baer AN, Christensen J, et al. Hypocomplementemic urticarial vasculitis syndrome. Clinical and serologic findings in 18 patients. Medicine (Baltimore) 1995;74:24-41. [Crossref] [PubMed]
- Schwartz HR, McDuffie FC, Black LF, et al. Hypocomplementemic urticarial vasculitis: association with chronic obstructive pulmonary disease. Mayo Clin Proc 1982;57:231-8. [PubMed]
- Paira SO. Bilateral pleural effusion in a patient with urticarial vasculitis. Clin Rheumatol 1994;13:504-6. [Crossref] [PubMed]
- Sakane T, Takeno M, Suzuki N, et al. Behcet’s disease. N Engl J Med 1999;341:1284-91. [Crossref] [PubMed]
- Erkan F, Gül A, Tasali E. Pulmonary manifestations of Behcet’s disease. Thorax 2001;56:572-8. [Crossref] [PubMed]
- Erkan F, Cavdar T. Pulmonary vasculitis in Behcet’s disease. Am Rev Respir Dis 1992;146:232-9. [Crossref] [PubMed]
- Castañer E, Alguersuari A, Gallardo X, et al. When to suspect pulmonary vasculitis: radiologic and clinical clues. Radiographics 2010;30:33-53. [Crossref] [PubMed]
- Chae EJ, Do KH, Seo JB, et al. Radiologic and clinical findings of Behcet disease: comprehensive review of multisystemic involvement. Radiographics 2008;28. [Crossref] [PubMed]
- Efthimiou J, Johnston C, Spiro SG, et al. Pulmonary disease in Behcet’s syndrome. Q J Med 1986;58:259-80. [PubMed]
- Hassine E, Bousnina S, Marniche K, et al. Pulmonary artery aneurysms in Behcet’s disease: contribution of imaging in 5 cases. Ann Med Interne (Paris) 2002;153:147-52. [PubMed]
- Emad Y, Abdel-Razek N, Gheita T, et al. Multislice CT pulmonary findings in Behcet’s disease (report of 16 cases). Clin Rheumatol 2007;26:879-84. [Crossref] [PubMed]
- Trad S, Bensimhon L, El Hajjam M, et al. 18F-fluorodeoxyglucose-positron emission tomography scanning is a useful tool for therapy evaluation of arterial aneurysm in Behcet’s disease. Joint Bone Spine 2013;80:420-3. [Crossref] [PubMed]
- Hatemi G, Yazici Y, Yazici H. Behcet’s syndrome. Rheum Dis Clin North Am 2013;39:245-61. [Crossref] [PubMed]
- Hamuryudan V, Er T, Seyahi E, et al. Pulmonary artery aneurysms in Behet syndrome. Am J Med 2004;117:867-70. [Crossref] [PubMed]
- Saadoun D, Asli B, Wechsler B, et al. Long-term outcome of arterial lesions in Behçet disease: a series of 101 patients. Medicine (Baltimore) 2012;91:18-24. [Crossref] [PubMed]
- Schreiber BE, Noor N, Juli CF, et al. Resolution of Behcet’s syndrome associated pulmonary arterial aneurysms with infliximab. Semin Arthritis Rheum 2011;41:482-7. [Crossref] [PubMed]
- Ceyran H, Akçali Y, Kahraman C. Surgical treatment of vasculo-Behcet’s disease. A review of patients with concomitant multiple aneurysms and venous lesions. Vasa 2003;32:149-53. [Crossref] [PubMed]
- Kalko Y, Basaran M, Aydin U, et al. The surgical treatment of arterial aneurysms in Behet disease: a report of 16 patients. J Vasc Surg 2005;42:673-7. [Crossref] [PubMed]
- Durieux P, Bletry O, Huchon G, et al. Multiple pulmonary arterial aneurysms in Behcet’s disease and Hughes-Stovin syndrome. Am J Med 1981;71:736-41. [Crossref] [PubMed]
- Rockall AG, Rickards D, Shaw PJ. Imaging of the pulmonary manifestations of systemic disease. Postgrad Med J 2001;77:621-38. [Crossref] [PubMed]
- Collard HR, Schwarz MI. Diffuse alveolar hemorrhage. Clin Chest Med 2004;25:583-92. [Crossref] [PubMed]
- de Prost N, Parrot A, Picard C, et al. Diffuse alveolar haemorrhage: factors associated with in-hospital and long-term mortality. Eur Respir J 2010;35:1303-11. [Crossref] [PubMed]
- Hellmich B, Ehlers S, Csernok E, et al. Update on the pathogenesis of Churg-Strauss syndrome. Clin Exp Rheumatol 2003;21:S69-77. [PubMed]
- Spira D, Wirths S, Skowronski F, et al. Diffuse alveolar hemorrhage in patients with hematological malignancies: HRCT patterns of pulmonary involvement and disease course. Clin Imaging 2013;37:680-6. [Crossref] [PubMed]
- Schnabel A, Holl-Ulrich K, Dalhoff K, et al. Efficacy of transbronchial biopsy in pulmonary vasculitides. Eur Respir J 1997;10:2738-43. [Crossref] [PubMed]
- Mukhtyar C, Guillevin L, Cid MC, et al. European Vasculitis Study Group. EULAR recommendations for management of primary small and medium vessel vasculitis. Ann Rheum Dis 2009;68:310-7. [Crossref] [PubMed]
- Travis WD, Colby TV, Lombard C, et al. A clinicopathologic study of 34 cases of diffuse pulmonary hemorrhage with lung biopsy confirmation. Am J Surg Pathol 1990;14:1112-25. [Crossref] [PubMed]


