The nationwide program of allergic disease prevention as an implementation of GARD guidelines in Poland
Introduction
Allergic diseases pose a serious problem for modern medicine and public health. In terms of epidemiology, they constitute the most important life-long disease in highly developed countries, as allergy affects between 10% and 50% of the population of Europe, the United States, Australia and New Zealand. This problem is reflected in documents, such as the Declaration adopted during the Polish Presidency in the European Union in 2011 (1,2). It is estimated that nearly 40% of the Polish population suffers from allergic diseases, including: allergic rhinitis (AR) 25%, signs and symptoms of asthma 12%, bronchial asthma (BA) confirmed by a medical examination and treated 5%, atopic dermatitis 9% and food allergy 13% (3-9).
In 2006, Global Initiative for Chronic Obstructive Lung Disease (GOLD) together with World Health Organization (WHO) established the Global Alliance against Chronic Respiratory Diseases (GARD) on the basis of the international ARIA group, to improve the health of the population affected by chronic diseases. The GARD strategy is based mainly on activities aimed at implementing preventive tasks at a local and global level, taking into account the similarities between chronic diseases (10,11).
The Act of 11 September 2015 on public health in Poland is of fundamental importance for state health policy. This act does not only define in a comprehensive way the duties of central and local (self-government) administration in the field of public health, but also provides for the principles of their financing (12,13). The most important implementing act is the National Health Program (NHP) adopted in the form of the Ordinance of the Minister of Health, but implemented by the forces of the entire government, which defines the strategy of the State in a five-year perspective. It sets six operational objectives selected for their impact on health of citizens, including limiting the health risk which results from physical, chemical and biological hazards in the external environment, work place, place of residence, places for recreation and study (14).
The Healthcare Institute was selected by the Ministry of Health as the implementer of the project “Research, development and promotion of issues related to risk factors of allergic diseases and asthma, especially of airborne origin”. This task is carried out in cooperation with the Medical University of Warsaw, Silvermedia Inc. (IT company specializing in medicine and e-health) and Onet.pl (the largest Polish-language website: 17 million users per month). The project is planned for 2017–2018. Its aim is to reduce the impact of allergic diseases on the health of children and adolescents at school age through education, screening, improving the availability of treatment and monitoring treatment results using modern ICT tools.
The project consists of two main parts:
- Screening of children and adolescents at school age;
- National education campaign.
The aim of the work is to present an example of effective implementation of GARD guidelines/recommendations in the area of prevention of allergic diseases.
Methods
Screening
According to the assumptions of the project, in 2017–2018, the screening will cover a group of at least 10,000 children and adolescents at school age (6–18 years of age) in 10 of 16 voivodships in Poland. In the study, a targeted sample selection based on school selection was used. In each voivodship, selected for participation in the project, cooperation with appointed educational institutions was established. Each child in a given school has an opportunity to take part in the screening test.
According to the assumptions of the project, the screening test is carried out with the use of e-health tools that have been previously verified in the pilot study.
The use of e-health tools for diagnostics, therapy support and monitoring of allergic diseases can significantly reduce the negative impact of these diseases both on the patient and the entire healthcare system. MACVIA-ARIA Sentinel NetworK for allergic rhinitis (MASK) is an example of this kind of comprehensive tele-information solutions. This solution has been developed by an international group of experts as an implementation of Action Plan B3 of the European Innovation Partnership on Active and Healthy Aging (EIP on AHA) (15). The part of MASK is the e-allergy algorithm, the version of which was also used in the described study.
Each person from the target group (or his/her parent/guardian) gains access to the project’s internet platform, which enables:
- Conducting screening with the use of an algorithm to assess the risk of allergic diseases;
- Obtaining individual recommendations for further treatment depending on the result of the screening test (algorithm);
- Getting access to the pollen calendar (taking into account regional diversity of the country);
- Searching for allergy clinics in the nearest neighborhood and obtaining information on waiting time (within the public health system).
All data obtained during tests are recorded in a central, anonymized database. Respondents are identified on the basis of the identification number assigned to them for the purpose of the following study.
Algorithm for early diagnosis of allergic diseases
In the study the e-Allergy algorithm for early premedical risk assessment of selected allergic diseases was used (15). The algorithm was developed using data from the ECAP project (18,617 effectively tested respondents, of whom 4,783 underwent additional outpatient tests) (3). Advanced statistical analysis methods, including neural networks and machine learning, were used to create it. For the first time, the algorithm was presented in 2011 at a conference of experts during the Polish Presidency of the EU Council (2). In the basic version, the algorithm assigns a given person to one of three risk groups (low, moderate or high) based on responses concerning different types of symptoms. The risk is determined separately for BA and AR. Depending on the risk group, a respondent receives recommendations for further proceedings. The respondent also receives a list of his/her answers regarding the occurrence of signs and symptoms of allergic diseases. This information is intended for a physician.
The survey used a questionnaire for the respondent (constituting a source of data for the operation of the algorithm) in electronic form. The questionnaire was composed of 42 questions, of which 38 related to the symptoms of allergic diseases and current treatment. The questions used in the questionnaire were mostly taken from the base of 453 questions of the ECAP project (which was based on the ECRHS II and ISAAC standards) (16,17). Based on the statistical analysis, the relationship between individual questions and selected allergic diseases was determined. The questions with the highest predictive power were used to develop the algorithm.
Some questions were conditional and only a part of respondents (with specific symptoms) were asked to answer them. The questions were closed or semi-open (with a possibility of adding answers). The time necessary to complete the questionnaire depended on the number of declared symptoms and in most cases accounted for between 5 and 10 minutes.
Validation of the research tool
A validation study was carried out before screening. The validation of the algorithm consisted in comparing the results (in the same person) of the diagnostic test (obtained through the use of an algorithm on questionnaire data) with the results of an outpatient examination for allergy.
It was a quantitative research. In the study the following research methods were used:
- Questionnaire survey (questionnaire in electronic form);
- Interview and physical examination;
- Skin prick test (SPT)—outpatient examination;
- Spirometry with reversibility test—outpatient examination.
The flow chart of the study is presented in Figure 1. It is in line with the so-called “diagnostic and clinical path” of the ECAP project (Epidemiology of Allergic Diseases in Poland; www.ecap.pl) (18).
The study was conducted in healthcare facilities providing paediatric allergy services and appropriate conditions for conducting the study. The outpatient part was carried out by a qualified medical personnel. Seventeen SPTs were used, including: white birch, grass/cereals, mugwort, alder, rye, plantain, cat’s fur, dog’s fur, Dermatophagoides pteronyssinus, Dermatophagoides farinae, Alternaria tenuis, chicken egg, cow’s milk, Cladosporium herbarum, histamine and control. In addition, a spirometry was carried out in each subject. In appropriate cases, a reversibility test was also performed (with salbutamol 400 µg).
The validation study was conducted on a group of 1,008 children and adolescents aged 6–18 from three urban centres (Warsaw, Poznan and Lodz). The percentage of boys in the study population constituted 51.2%. The selection of respondents was based on accessibility and the sample was not representative of the population.
The study was approved by the Bioethics Committee of the Medical University of Warsaw (KB/160/2017). Each person participating in the study expressed informed consent, which was confirmed by signing an appropriate statement.
Calibration of the algorithm
On the basis of the additional data collected during the validation study, the algorithm was calibrated to improve its effectiveness. The method of assigning respondents was also modified, instead of three risk groups there are two groups (probably healthy subjects and probably sick subjects), which is the standard solution for screening tests. In this way, it was possible to determine the sensitivity and specificity of the diagnostic test.
In order to achieve high accuracy with limited overfitting, the problem was treated as training four independent machine learning classification models. Firstly, for each type of illness, a group of suitable and anyhow connected questions was identified. Then, if they had a structure or logic behind, they were suitably converted. For instance, if an answer to one question could be derived from the previous ones, they were together transformed into one number. That was simply done by assigning some weights to each question’s answer and summing the weights from particular associated groups of questions.
Such preprocessed records were then analyzed to identify inconsistencies. That is to closely look into the contrary records (similar questions results with different categories associated). Then, the standard machine learning techniques could be employed. Usage of Random Forest (19) algorithms gave satisfactorily high accuracy. Also, the nature of this algorithm guarantees relatively low overfitting as each tree of the forest is constructed independently from a randomly generated sample of training records. The randomness also takes place in the way how each of the tree is trained, i.e., on which questions it should be concentrated. Finally, the model is an ensemble of multiple trees (in our case 500 trees) and the final illness probability is defined as the fraction of the trees in ensemble that classify the record as ill. The four independent Random Forest models were then analyzed in order to define the real accuracy.
Information and education campaign
The information and education campaign is carried out in 2017–2018. The main target group is parents and guardians of children and adolescents at school age (6–18 years), as well as teaching staff. Some of the activities are addressed directly to children (e.g., brochures). This nationwide campaign is implemented through:
- Distribution of information and education materials (guides, brochures);
- Informational and educational meetings;
- Publications in electronic media;
- Onet.pl (internet portal that reaches nearly 64% of Polish internet users);
- YouTube (the largest website that allows free placement and playing of videos);
- Social media sites: Onet Lifestyle, Onet Woman, Medonet and Homepage Onet.pl.
According to the assumptions of the project, 600,000 unique users of electronic media will be familiarized with information and educational materials by the end of the project (end of 2018).
Results
Due to the fact that at the time of writing this article the project is still implemented, it is not possible to describe all its results. The preliminary results of the project achieved in 2017 are presented below.
Validation of the research tool
A total of 1,008 children and adolescents from the target group participated in the validation study. In outpatient examination AR was diagnosed in 46.4% and BA in 11.2%. The BA and AR risk assessment based on the original version of the algorithm is presented in Figure 2.
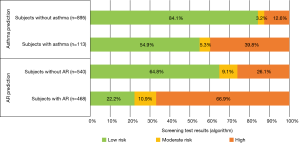
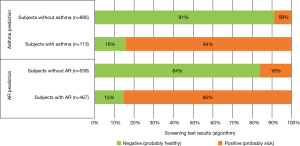
On the basis of the data obtained during the validation study, the algorithm was calibrated to better match the specificity of the target group. The standard screening test division into two groups: probably healthy (negative result) and probably sick (positive result) was also adopted. Therefore, it was possible to determine the sensitivity and specificity of the new version of the algorithm. In the case of AR, the sensitivity of the diagnostic test was 0.852 and the specificity was 0.840. In the case of BA, it was 0.841 and 0.912, respectively. The detailed classification method is presented in Figure 3.
Screening
For the purpose of the screening test, the online platform of mAlergia (www.mAlergia.pl) was created, providing users with access to the above-mentioned functions (Figure 4).
On the platform, various types of information materials concerning allergic diseases and methods of their diagnosis and treatment were published. All Internet users can access the content of the platform. The only exception is the screening module, which is available only for the target group (an access code is required).
The web platform was launched in the second half of 2017. In total, 1,512 people used the screening tool in 2017, of which 1,472 respondents went through all stages of the assessment. Among people who used the screening tool, the positive AR result (probably sick) was found in 19.5% of respondents (18.1% in boys, 20.6% in girls), and BA in 8.4% (8.4% regardless of gender). The remaining people from the target group will be examined by the end of 2018.
Information and education campaign
The main part of the information and education campaign is carried out through the Onet.pl online portal. At http://alergie.onet.pl/, a content hub has been created – a special website in which materials developed for the purposes of the project are published (Figure 5). Publications are prepared by the editors of Onet.Zdrowie and Medonet. The content presented on thematic websites is promoted by the editors of Onet.Kobieta and social media websites related to Onet.pl. As part of the project, articles, infographics, quizzes, knowledge tests, short videos in Onet100 format (Figure 6) and episodes of video programs "Recipe for health" are published. The whole project is complemented with a display campaign broadcast on the entire Onet—RASP (one of the biggest multimedia companies in Poland) network.

In 2017, 22 different types of materials were published as part of the information and education campaign. In addition, 25 editorial posts were published on various social media websites (Facebook).
Traditional mass media were also used to support the project. In 2017, one press conference was organized and two information brochures on allergic diseases for children and adolescents and their parents/guardians were sent to schools (Figure 7).
Discussion
Due to the comprehensiveness of activities carried out under NHP, the programme is consistent with the main objective of GARD: “to develop a comprehensive approach for the surveillance, diagnosis, prevention and control of chronic respiratory diseases and integrated programme of chronic diseases prevention” (10,11). On the one hand, it is an universal tool enabling development of standard procedures for obtaining reliable data on chronic respiratory diseases (10) and on the other hand it is an attempt to implement a developed model of prevention activities as a part of the locally conducted screening program. Due to the significant incidence of chronic respiratory diseases, it seems reasonable to implement population screening programs tailored to the local needs, enabling early prevention to minimize long-term health effects (10,11). The effectiveness of screening activities in the prevention of allergic diseases is confirmed by numerous publications using questionnaires—clinically useful and reliable tools in diagnosing AR and assessing the severity of BA. For example, Hojo et al. assigned the SACRA questionnaire (State of the Impact of Allergic Rhinitis on Asthma Control) high usefulness not only in the context of diagnosis but also monitoring of the therapy effectiveness. The specificity and sensitivity of SACRA accounted for 92.4% and 65.7%, respectively in comparison to the standard ARIA questionnaire −89.2% and 76.7% (20).
The implementation of NHP in the area of early diagnosis and prevention of allergic diseases was carried out based on the described above project, including the application (implementation) of modern screening techniques, mass screening and the information and education campaign raising awareness about allergies that can occur regardless of age.
The results of the validation study showed satisfactory effectiveness of the algorithm in relation to AR, whereas in case of BA the effectiveness was definitely lower. This may be partly due to the fact that the algorithm was based on data from the ECAP project, the main part of which was implemented in 2006–2008. In the meantime, the consciousness/knowledge of the society concerning allergic diseases could have changed, which may have an impact on answers to the questionnaire. It seems, however, that the age of the respondents was more important. The ECAP project was conducted among three age groups: children 6–7 years of age, adolescents 13–14 years of age, and young adults (20–44 years of age). Therefore, the algorithm developed on the basis of data from such a group could show some mismatch in relation to the target group of the project (children and adolescents aged between 6 and 18 years). New data obtained as part of the project allowed for the calibration of the algorithm, which significantly increased its effectiveness.
Limitations and strengths of the project
The limitations of the project include using mainly new media for the purposes of the information and education campaign, which due to their nature may constitute a barrier for people who do not use the Internet or do it rarely. However, according to Polish Public Opinion Research Center estimates, in 2017, 67% of Polish citizens used the Internet at least once a week. Among people aged between 18 and 44 years, the percentage of Internet users ranged from 87% to 100% (21).
The recruitment system is also a limitation of the study. All students from a given educational institution are invited, but the participation is completely voluntary. Due to the fact that no data is collected on people who refused to participate in the study, it is impossible to determine whether the refusals are random or systematic. Thus, the collected data should not be used in epidemiological descriptions of the entire population, due to the fact that it is impossible to assess the representativeness of the sample.
Another limitation is the impossibility to verify under the project a preliminary diagnosis (result of a diagnostic test) made by the algorithm during the screening test. Subjects with a positive test result are referred to a primary care physician and then to an allergist. However, the medical examination takes place under the public health system (not as part of this project).
The strengths of the project include low unit costs. This is the result of using a screening system based on e-health technology. Another advantage of the technology used is that the algorithm provides fast results. After the completion of the research questionnaire, the subject immediately receives the result of the diagnostic test (algorithm) and recommendations regarding further procedures. Very important is the open access to this type of screening test, limited only by the ability to use the Internet. Obtaining information on the allergy risk with a list of responses to the questionnaire, helps on the one hand to indicate a potential patient if there is a need for further diagnostics of allergic diseases, and on the other hand to provide an accurate diagnosis by the physician. The answers provided in the screening test help to understand the patient's efforts to see a specialist.
The complexity of the project is also its strength. The project combines allergy screening with a nationwide information and education campaign. Both parts of the project support each other.
The use of new media as the main channel of communication can also be considered as a strength of the project. Using the Internet to communicate with the target group reduces the costs and allows for the use of more interactive materials in comparison to traditional methods. Moreover, the Internet is the main source of information for the target group (22).
Considering the prevalence of allergy and asthma, the following project constitutes a significant progress in the education, prevention and treatment of the above-mentioned diseases in Poland, especially in the field of pre-medical diagnosis, as well as presentation of evidence-based knowledge in media.
The NHP implemented in Poland on the basis of the above project requires further evaluation in terms of the effectiveness of improving the health status of the population in the area of allergy and asthma. The applied methods should be used for a longer time period to enable the critical evaluation of their effectiveness and, if necessary, to improve them in order to achieve appropriate effects.
Conclusions
- The internet media and e-health tools create new opportunities for implementing the GARD strategy, focusing on the improvement of the health of the population affected by chronic respiratory diseases. The project on allergic diseases implemented in Poland under NHP is an example of effective implementation of this concept.
- Despite non-random sampling, the obtained screening results indicate the persistent high prevalence of allergy and asthma.
- Epidemiological data indicate the necessity of conducting population screening programmes for allergic diseases.
- The e-health tools should be used in screening (pre-medical diagnosis). Their sensitivity and specificity is sufficient and they are significantly cheaper than other methods.
- Screening should be complemented with a mass-scale information and education campaign, which allows to achieve a synergistic effect.
Acknowledgements
The authors would like to thank all those involved in the project.
Funding: The project is financed by the Minister of Health (number 6/4/2.2.1.3/NPZ/3179/621).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Bioethics Committee of the Medical University of Warsaw (No. KB/160/201) and written informed consent was obtained from all patients.
References
- Samoliński B, Fronczak A, Włodarczyk A, et al. Council of the European Union conclusions on chronic respiratory diseases in children. Lancet 2012;379:e45-6. [Crossref] [PubMed]
- Samoliński B, Fronczak A, Kuna P, et al. Prevention and control of childhood asthma and allergy in the EU from the public health point of view: Polish Presidency of the European Union. Allergy 2012;67:726-31. [Crossref] [PubMed]
- Sybilski AJ, Raciborski F, Lipiec A, et al. Atopic derm atitisis a serious health problem in Poland. Epidemiology studies based on the ECAP study. Postepy Dermatol Alergol 2015;32:1-10. [Crossref] [PubMed]
- Dulny G, Sybilski AJ, Zalewska M, et al. The Effect of Preventive Immunization on the Incidence of Allergic Conditions. Iran J Allergy Asthma Immunol 2015;14:402-9. [PubMed]
- Sybilski AJ, Zalewska M, Furmańczyk K, et al. The prevalence of sensitization to inhalant allergens in children with atopic dermatitis. Allergy Asthma Proc 2015;36:e81-5. [Crossref] [PubMed]
- Sybilski AJ, Raciborski F, Lipiec A, et al. Atopic dermatitis is a serious health problem in Poland. Epidemiology studies based on the ECAP study. Postepy Dermatol Alergol 2015;32:1-10. [Crossref] [PubMed]
- Sybilski AJ, Lusawa A, Lipiec A, et al. The effects of disease awareness on lifestyle changes and the use of preventive measures in asthma patients. Allergy Asthma Proc 2015;36:e14-22. [Crossref] [PubMed]
- Sybilski AJ, Raciborski F, Lipiec A, et al. Epidemiology of atopic dermatitis in Poland according to the Epidemiology of Allergic Disorders in Poland (ECAP) study. J Dermatol 2015;42:140-7. [Crossref] [PubMed]
- Sybilski AJ, Raciborski F, Lipiec A, et al. Obesity--a risk factor for asthma, but not for atopic dermatitis, allergic rhinitis and sensitization. Public Health Nutr 2015;18:530-6. [Crossref] [PubMed]
- Bousquet J, Dahl R, Khaltaev N. Global Alliance against Chronic Respiratory Diseases Pneumonol Alergol Pol 2008;76:160-9. [original title: Światowy Sojusz Przeciwko Przewlekłym Chorobom Układu Oddechowego]. [PubMed]
- Khaltaev N. GARD, a new way to battle with chronic respiratory diseases, from disease oriented programmes to global partnership. J Thorac Dis 2017;9:4676-89. [Crossref] [PubMed]
- The Act of 11 September 2015 on public health, Journal of Laws of 2015, item 1916. Available online: http://prawo.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20150001916
- Grudziąż-Sękowska J, Sękowski K, Cianciara D. Public Health Act - new standards? Post N Med 2016;XXIX:337-43.
- The Ordinance of the Council of Ministers of 4 August 2016 on the National Health Program 2016-2020, Journal of Laws of 2016, item 1492. Available online: http://prawo.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20160001492
- Bousquet J, Schunemann HJ, Fonseca J, et al. MACVIA-ARIA Sentinel NetworK for allergic rhinitis (MASK-rhinitis): the new generation guideline implementation. Allergy 2015;70:1372-92. [Crossref] [PubMed]
- The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema Lancet 1998;351:1225-32. [Crossref] [PubMed]
- European Community Respiratory Health Survey: Variations in the prevalence of respiratory symptoms, self-reported asthma, and use of asthma medication in the European Community Respiratory Health Survey. Eur Respir J 1996;9:687-95. [Crossref] [PubMed]
- ECAP web page. Available online: . Acces:07.08.2018.www.ecap.pl
- Breiman L. Random Forests. Machine Learning 2001;45:5-32. [Crossref]
- Hojo M, Ohta K, Iikura M, et al. Clinical usefulness of a guideline based screening tool for the diagnosis of allergic rhinitis in asthmatics: The Self Assessment of Allergic Rhinitis and Asthma questionnaire. Respirology 2013;18:1016-21. [Crossref] [PubMed]
- Public Opinion Research Center, Research report No. 49/2017 7. Available online: . Access: 3.08.2018.https://www.cbos.pl/SPISKOM.POL/2017/K_049_17.PDF
- Kłak A, Raciborski F, Samoliński B. Searching online for health-related information by people suffering from respiratory allergy and asthma: the results of a survey study. Adv Respir Med 2017;85:87-96. [Crossref] [PubMed]