Use of veno-venous extracorporeal life support in a patient with cytomegalovirus and Pneumocystis jiroveci related respiratory failure due to thymoma associated immunodeficiency: a case report
Introduction
Although the use of extracorporeal life support (ECLS) treatment is increasing rapidly, its use in immunocompromised patients is still under debate as infections are a main predictor of poor outcome during ECLS therapy. Predicting the prognosis for a specific type of immunocompromised patient is challenging and is depending on the underlying disease and the amount of accumulated organ damage that has occurred.
We report a case of initial successful ECLS treatment in a patient with severe respiratory failure due to cytomegalovirus and Pneumocystis jiroveci with severe thymoma associated B cell deficiency (Good’s syndrome), T cell deficiency and multiorgan autoimmunity.
Case presentation
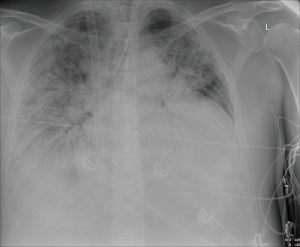
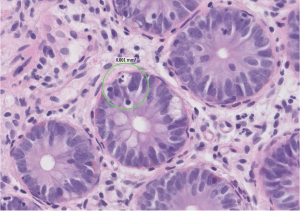
A 55-year-old man with a recent diagnosis of inflammatory bowel disease and an incidentally detected thymoma (type A, Masaoka stadium 1, pT1a) was admitted with respiratory failure and shock to the intensive care unit (ICU), where he was intubated and mechanically ventilated, received fluid resuscitation and vasopressor support. The bronchial-alveolar lavage determined cytomegalovirus and Pneumocystis jiroveci (positive DNA with PCR) as underlying cause and treatment with trimethoprim/sulfamethoxazole and ganciclovir was started. Laboratory results showed a severe hypogammaglobulinemia, with absence of circulating B cells and therefore the diagnosis of Good’s syndrome was considered (1,2). As respiratory failure worsened he was referred to our hospital for ECLS treatment and thymectomy. On admission the PaO2/FiO2 ratio was 66 mmHg with a respiratory acidosis (pH 7.19, pCO2 10.3 kPa) on pressure controlled mechanical ventilation with a respiratory rate of 20, inspiratory pressure of 35 cmH2O and PEEP 16 cmH2O. The Resp score was −2, predicting to a survival of 40% (3). Therefore after 11 days of mechanical ventilation veno-venous ECLS treatment was initiated (Figure 1). Antibiotic and antiviral therapy was continued and immunoglobulins were administered to maintain a level of ≥5 g/L. During the 4 weeks on ECLS treatment recurrent infections were successfully treated, however, the patient developed diffuse erythema and continued to have malabsorption. Repeated biopsies of the gut and skin were performed which demonstrated active immune inflammation characterized by apoptotic bodies and T cell infiltrates, without signs of infectious agents (Figure 2). After 4 weeks a medullary biopsy was performed which, in combination with laboratory results (lymphocytes 0.15×109/L, no circulating B-cells CD19, T-cells CD8: 0.07×109/L, T-cells CD8: 0.03×109/L, NK-cells CD16+CD56+: 0.04×109/L) and skin and gut biopsies, revealed that he was suffering from thymoma-associated multiorgan autoimmunity (TAMA). TAMA is a thymoma-associated B and T cell immunodeficiency with secondary immune dysregulation resulting in dermatitis and enteropathy (4). High dose pulse therapy methylprednisolone was started and 1 week later ECLS treatment could be terminated and patient underwent a thymoma resection. After weaning from mechanical ventilation patient was dismissed from the ICU within two weeks of surgery. He was treated with cyclophosphamide, cyclosporine and prednisolone to control his immune dysregulation. Unfortunately, 50 days after ICU discharge, but still on the ward, he had an intraparenchymal frontal bleeding. Further treatment was considered futile and the patient died.
Discussion
We report a case of initially successful ECLS treatment in a patient with Good’s syndrome and TAMA. The presence of a thymoma in combination with a primary immunodeficiency syndrome characterized by hypogammaglobulinemia and the absence of peripheral B cells, is compatible with the diagnosis of Good’s syndrome that occurs in approximately 5% of patients with a thymoma (1,2). When viral infections, opportunistic infections, and chronic diarrhea are present as well a more widespread immune deficiency with immune dysregulation should be considered. In our patient biopsies of skin, gut and thymoma ultimately led to the additional diagnosis of TAMA. TAMA is a rare paraneoplastic disorder, clinically and pathologically similar to graft-versus-host disease (4). The disease has an unfavorable clinical course needing intensive immunosuppressant therapy, especially in case of an unresectable thymoma, and gives graft-versus-host disease-like symptoms and accompanying autoimmune diseases (5). The risk of additional immune suppressive therapy needed to treat the TAMA is greatly increased in patients in whom opportunistic infections are already present. The use of ECLS in this specific condition is unique as far as we know but may be compared with ECLS therapy in patients with respiratory failure after allogenic hematopoietic stem cell transplantation. A study in 37 patients after allogeneic hematopoietic stem cell transplantation showed that only seven patients (19%) survived to hospital discharge. Of the five patients (14%) in which acute graft-versus-host disease was present none survived (6). Our experience confirms the high mortality risk of immunocompromised patients with severe respiratory failure on ECLS. Although ECLS therapy may be helpful to treat the acute respiratory failure as in our case it only serves as a bridge to potential recovery. We must realize that in these patients with a poor risk due to the underlying disease the long-term changes for survival may be very low. Treatment decisions must be based on individual patient characteristics among which the underlying immunodeficiency.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Kelleher P, Misbah SA. What is Good's syndrome? Immunological abnormalities in patients with thymoma. J Clin Pathol 2003;56:12-6. [Crossref] [PubMed]
- Kelesidis T, Yang O. Good's syndrome remains a mystery after 55 years: A systematic review of the scientific evidence. Clin Immunol 2010;135:347-63. [Crossref] [PubMed]
- Schmidt M, Bailey M, Sheldrake J, et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am J Respir Crit Care Med 2014;189:1374-82. [Crossref] [PubMed]
- Wadhera A, Maverakis E, Mitsiades N, et al. Thymoma-associated multiorgan autoimmunity: a graft-versus-host-like disease. J Am Acad Dermatol 2007;57:683-9. [Crossref] [PubMed]
- Bernard C, Frih H, Pasquet F, et al. Thymoma associated with autoimmune diseases: 85 cases and literature review. Autoimmun Rev 2016;15:82-92. [Crossref] [PubMed]
- Wohlfarth P, Beutel G, Lebiedz P, et al. Characteristics and Outcome of Patients After Allogeneic Hematopoietic Stem Cell Transplantation Treated With Extracorporeal Membrane Oxygenation for Acute Respiratory Distress Syndrome. Crit Care Med 2017;45:e500-7. [Crossref] [PubMed]


