Interstitial lung disease associated with anti-citrullinated peptide/protein antibody-positive anti-synthetase syndrome
Introduction
Assays for detecting anti-citrullinated peptide/protein antibody (ACPA) have been shown to be a very good diagnostic tool for rheumatoid arthritis (RA) due to their its high specificity (1). On the other hand, anti-aminoacyl-tRNA synthetase (anti-ARS) antibodies are the most frequently detected in anti-synthetase syndrome (ASS) (2). Most clinical manifestations of ASS are characterized by myositis [i.e., polymyositis (PM)/dermatomyositis (DM)], arthritis, mechanic’s hand, interstitial lung disease (ILD), and Raynaud’s phenomenon (2-4). Serological testing of autoantibodies of ILD patients is recommended to identify the high proportion of patients with connective tissue disease (CTD)-ILD with implications for diagnosis and management (5). Recently defined “interstitial pneumonia with autoimmune features (IPAF)” also emphasizes the importance of CTD-related autoantibodies (6). On the other hand, we previously suggested that each of the specific autoantibodies of IPAF may be necessary to assess the appropriate strategy to diagnose and treat IPAF (7). The features of ILD associated with ACPA and anti-ARS were previously reported (8-10). However, even though a few cases have been reported (11-14), the clinical significance of ILD associated with ACPA-positive ASS has not yet been evaluated. Our aim was to review ILD associated with ACPA-positive ASS patients. Whether ILD associated with ACPA-positive ASS should be managed in accordance with ACPA-positive ILD or ASS-associated ILD is a large clinical question. This investigation was clinically relevant and important to determine appropriate treatment of ILD associated with ACPA-positive ASS patients.
Methods
Materials
Our study included 71 consecutive patients diagnosed with ASS-associated ILD between December 2010 and May 2016 at our hospital. These patients were carried out routine examination of ACPA. Of those, we selected 7 consecutive patients diagnosed as having ILD associated with ACPA-positive ASS. This retrospective cohort study was approved by the institutional review board of Kanagawa Cardiovascular and Respiratory Center (KCRC-17-0014). Because of the retrospective nature of the study, the review board waived the need for written informed consent from the patients.
Study methods
Baseline clinical measurements were obtained within 3 months of the initial diagnosis of ILD. ACPA was tested using chemiluminescent enzyme immunoassay testing of sera (BML Inc., Japan). A positive result for ACPA was defined as a measurement greater than 4.5 U/mL. For anti-ARS antibodies (anti-Jo-1, EJ, PL-7, PL-12, OJ, and KS antibodies), routine conserved serum of each patient at the diagnosis of ILD in Kanagawa Cardiovascular and Respiratory Center was measured by RNA immunoprecipitation and protein immunoprecipitation assays at Tokai University School of Medicine, Japan. ASS patients were defined as having one of the ARS antibodies with ILD. Two experienced thoracic radiologists (T Iwasawa and S Iso) reviewed all high-resolution computed tomography (HRCT) scans for consensus of diagnosis of ILD in our hospital without knowledge of the patients’ clinical data. The HRCT pattern was based on the previous guidelines (15). Findings inconsistent with the usual interstitial pneumonia (UIP) pattern were classified into nonspecific interstitial pneumonia (NSIP), organizing pneumonia (OP), and NSIP with OP overlap (NSIP + OP) according to previous reports (16,17). NSIP + OP was identified when consolidations were superimposed on a background of ground-glass opacity (GGO) with or without reticulations or traction bronchiectasis (16). HRCT findings were analyzed from each of the characteristic viewpoints of ACPA-positive ILD (i.e., honeycombing, centrilobular nodules, bronchial wall thickening, and expiratory air trapping) and ILD associated with ASS (i.e., lower lung volume loss and lower lung predominance) referring to previous reports (9,10,16,18,19). These HRCT findings were interpreted according to the recommendations of the Fleischner Society (20). Disagreements between the two radiologists after the initial assessment were resolved by discussion. Surgical lung biopsy specimens were available from 3 patients and were reviewed by two pulmonary pathologists (K Okudela and T Takemura) who were blinded to the patients’ clinical and radiologic information. Histologic patterns were classified according to the classification of idiopathic interstitial pneumonia (17,21). Disagreements between the two pathologists were discussed until consensus was reached. Treatment responses as measured by pulmonary function tests are presented as the percentage change of the initial value. Improved and Worsened were defined as >10% positive or negative changes, respectively, in forced vital capacity (FVC) or >15% in %diffusing capacity of the lung for carbon monoxide (%DLCO) (22). Patients who did not meet the criteria for consideration as Improved or Worsened were considered Stable.
Results
Patient characteristics
In our cohort of ASS-associated ILD, 7 patients (9.9%) were diagnosed ILD associated with ACPA-positive ASS. A summary of the characteristics of the patients with ILD associated with ACPA-positive ASS in our study is shown in Table 1. Of the 7 patients, 6 (85.7%) were female, and 4 (57.1%) had a history of smoking. Median patient age was 67 years (range, 51–87 years). Anti-ARS antibodies included anti-Jo-1 in 4 patients (57.1%), anti-PL-7 in 1 (14.3%) patient, anti-KS in 1 (14.3%) patient, and anti-EJ in 1 (14.3%) patient. Clinical symptoms of ASS other than ILD included arthralgia in 3 patients (42.9%), myalgia or muscle weakness in 2 patients (28.6%), mechanic’s hand in 4 patients (57.1%), Raynaud’s phenomenon in 1 patient (14.3%), and Gottron’s sign in 1 patient (14.3%). Pulmonary function test results showed a median %FVC of 73.8% (range, 60.9–81.1%) and %DLCO of 61.3% (range, 35.8–69.8%).

Full table
Radiological and pathological analysis
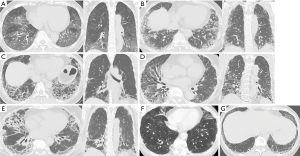
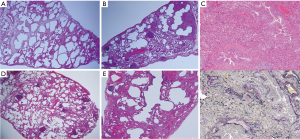
HRCT in 5 patients (71.4%) revealed GGO or consolidation along the bronchovascular bundles, which indicated NSIP in 3 patients (cases 1, 4, 5) and NSIP + OP in 2 patients (cases 2, 3) (left panel in Figure 1A,B,C,D,E). Coronal slices from these 5 patients showed lower lung predominance and major fissures being dragged caudally due to lower lobe volume loss (right panel in Figure 1A,B,C,D,E). In addition, the HRCT pattern included OP in 1 patient (case 6) (Figure 1F) and possible UIP in the other patient (case 7) (Figure 1G). The pathological findings in 3 cases resulted in a diagnosis of fibrotic NSIP in 2 patients (cases 1, 7) and cellular NSIP in 1 patient (case 3) (Figure 2). Additionally, lymphoid follicles with cellular infiltration were also seen in 2 cases (case 3, 7). With regard to airway disease, cellular bronchiolitis were seen with NSIP in 1 patient (case 3).
Treatment response and outcome
Six (85.7%) of the patients with ILD associated with ACPA-positive ASS received anti-inflammatory drugs (prednisolone + cyclosporine or tacrolimus) within 3 months after the ILD diagnosis, and the other patient (case 7) was carefully observed. After starting therapy, muscle or joint symptoms of ASS other than ILD improved in all 3 patients. In addition, no one developed these symptoms during follow-up period. All 6 patients who received anti-inflammatory therapy showed radiological improvement of their ILD. Of the 4 patients with available pulmonary function test results during follow-up, treatment responses resulted in 3 patients being considered Improved and 1 patient being considered stable. The median follow-up period was 34 months (range, 14–56 months), during which only 1 patient (case 4) died due to chronic mild progressive ILD and old age (Table 1).
Discussion
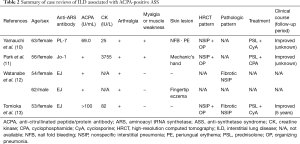
From the viewpoint of radiological features, our study suggested that the characteristics of ILD associated with ACPA-positive ASS may appear similar to those of ILD associated with ASS, but not those of RA or ACPA. As in previous reports of ILD associated with ASS (10,16), our patients with ILD associated with ACPA-positive ASS also showed that the most common HRCT pattern was NSIP, followed in order by NSIP + OP, possible UIP, and OP. Moreover, the patients in 3 previous case reports of ILD associated with ACPA-positive ASS presented with NSIP + OP (11,12,14) (Table 2). Coronal slices of our 5 cases (cases 1–5) showed lower predominance of reticulation, GGO, and traction bronchiectasis with or without consolidation, which indicated that lower lobe volume loss resulted in dragging major fissures caudally. These specific findings were reported to be characteristic of ILD associated with ASS by Fischer et al. (19). However, the lung phenotypic characteristics resemble those of RA-ILD and ACPA-positive ILD but not RA [8]. In RA-ILD or ILD associated with ACPA, unlike in most other CTDs, the UIP pattern is more commonly seen than NSIP (8,23). Additionally, no previous reports showed a NSIP + OP pattern in RA-ILD including ILD associated with ACPA. Only 1 patient (case 7) showed a possible UIP pattern in our study. Taken together, the results of our patients and previous case reports suggested the possibility that ACPA-positive ASS favors a characteristic radiological ILD pattern of ASS rather than ILD associated with RA or ACPA.
Full table
The most common histological pattern of CTD-ILD is NSIP (24). In contrast, the proportion of UIP pattern was reported to be higher in ILD associated with RA or ACPA followed by NSIP (8,25). Although it was previously thought in the past that lymphoid follicles are commonly seen especially in ILD associated with RA or ACPA, lymphoid follicles were a remarkable finding in ILD associated with ASS as reported by Watanabe et al. (13). Our previous pathological analysis of anti-EJ antibody associated ILD also showed lymphoid follicles in half of the cases (26). Our cases with available pathological findings showed NSIP with or without lymphoid follicles, and previous case reports also showed the same (Table 2) (13,14). On the other hand, high ACPA titer was significantly associated with small airway disease in RA subjects (18). Although only 1 patient (case 3) showed cellular bronchiolitis with ILD, coexistence of small airway disease and ILD may be meaningful in ACPA-positive ASS patients. In other words, from the viewpoint of pathological findings, we could not determine whether ILD associated with ACPA-positive ASS is closer in character to ILD associated with ASS or ACPA.
The clinical symptoms of some of the ACPA-positive ASS patients included severe arthritis, but those patients with myositis showed a good treatment response (27,28). If the patient have arthralgia or arthritis, these symptoms are hard to decide if it is either RA or ASS (28). Meyer et al. reported that approximately 40% of ACPA-positive ASS patients were still under prednisolone treatment at a dosage ≥10 mg/day (mean follow-up, 93.19 months) (28). None of our 3 patients (case 1–3) experienced a relapse of arthralgia under prednisolone (≤10 mg/day) during the follow-up period. Although only a few cases were analyzed, combined therapy with tacrolimus as a calcineurin inhibitor might also be effective. The reported treatment response of ILD of most patients, whether those with NSIP (including NSIP + OP) in association with ASS or CTD (e.g., RA, PM/DM) was often good as in our patients (16,21,29).
Recent guidelines recommend serologic evaluations such as rheumatoid factor, ACPA, and anti-nuclear antibody in ILD, even in the absence of signs or symptoms of CTD, provided that more specific antibodies such as anti-ARS antibodies are evaluated in select cases because of unclear diagnostic value of the other evaluations and to improve cost effectiveness (15). However, anti-TNF agents for RA may even trigger myositis and/or ILD in ASS and lead to refractory arthritis (27,30). In other words, RA therapy may lead to harmful events (including ILD) in patients diagnosed as having RA when the patient’s ILD is associated with ACPA-positive ASS. Therefore, we may perform serologic tests for anti-ARS antibodies in ILD patients even if they are positive for ACPA. Particularly, special care should be taken when assessing patients with a radiological ILD pattern of ASS rather than RA or ACPA, as described above.
Our results need to be interpreted with caution due to the following limitations. First, this was a retrospective, the sample size was small, and some clinical and pathological findings were not available. Second, since ILD became the reason why our patient was diagnosed as ACPA-positive ASS, this study was viewed from respiratory physicians, not rheumatologists. However, if the patients have autoantibodies related to CTD, we usually consult the rheumatologists to determine whether the diagnosis of CTD can be fulfilled. Third, there was selection bias because not all of the patients with ILD were evaluated for ACPA or anti-ARS antibodies, there was selection bias. However, the chest clinicians in our center carry out screening while always keeping routine examination of each antibody in mind, even if the patients with ILD have no symptoms suspicious of CTD. Therefore, we could collect rare cases of ILD associated with ACPA-positive ASS.
We conclude that despite these limitations, our study suggests that the characteristics of ILD associated with ACPA-positive ASS are similar to those of ILD associated with ASS. We believe that the results of our study will be helpful in determining the management of ILD, particularly in terms of serologic evaluation. Further studies are warranted to determine appropriate treatment of ILD associated with ACPA-positive ASS.
Acknowledgements
We sincerely thank Ms. Etsuko Iwata and Ms. Miyako Nakagawa of Tokai University School of Medicine for their contribution in detecting the anti-aminoacyl tRNA synthetase antibodies. The authors would like to thank Rise Japan LLC for the professional English language review.
Footnote
Conflicts of Interest: All work was performed at the Kanagawa Cardiovascular and Respiratory Center. The authors have no conflicts of interest to declare.
References
- Avouac J, Gossec L, Dougados M. Diagnostic and predictive value of anti-cyclic citrullinated protein antibodies in rheumatoid arthritis: a systematic literature review. Ann Rheum Dis 2006;65:845-51. [Crossref] [PubMed]
- Nakashima R, Imura Y, Hosono Y, et al. The multicenter study of a new assay for simultaneous detection of multiple anti-aminoacyl-tRNA synthetases in myositis and interstitial pneumonia. PLoS One 2014;9. [Crossref] [PubMed]
- Aggarwal R, Cassidy E, Fertig N, et al. Patients with non-Jo-1 anti-tRNA-synthetase autoantibodies have worse survival than Jo-1 positive patients. Ann Rheum Dis 2014;73:227-32. [Crossref] [PubMed]
- Hervier B, Devilliers H, Stanciu R, et al. Hierarchical cluster and survival analyses of antisynthetase syndrome: phenotype and outcome are correlated with anti-tRNA synthetase antibody specificity. Autoimmun Rev 2012;12:210-7. [Crossref] [PubMed]
- Raghu G. Idiopathic pulmonary fibrosis: guidelines for diagnosis and clinical management have advanced from consensus-based in 2000 to evidence-based in 2011. Eur Respir J 2011;37:743-6. [Crossref] [PubMed]
- Fischer A, Antoniou KM, Brown KK, et al. An official European Respiratory Society/American Thoracic Society research statement: interstitial pneumonia with autoimmune features. Eur Respir J 2015;46:976-87. [Crossref] [PubMed]
- Yamakawa H, Hagiwara E, Kitamura H, et al. Clinical Features of Idiopathic Interstitial Pneumonia with Systemic Sclerosis-Related Autoantibody in Comparison with Interstitial Pneumonia with Systemic Sclerosis. PLoS One 2016;11. [Crossref] [PubMed]
- Fischer A, Solomon JJ, du Bois RM, et al. Lung disease with anti-CCP antibodies but not rheumatoid arthritis or connective tissue disease. Respir Med 2012;106:1040-7. [Crossref] [PubMed]
- Takato H, Waseda Y, Watanabe S, et al. Pulmonary manifestations of anti-ARS antibody positive interstitial pneumonia--with or without PM/DM. Respir Med 2013;107:128-33. [Crossref] [PubMed]
- Waseda Y, Johkoh T, Egashira R, et al. Antisynthetase syndrome: Pulmonary computed tomography findings of adult patients with antibodies to aminoacyl-tRNA synthetases. Eur J Radiol 2016;85:1421-6. [Crossref] [PubMed]
- Yamauchi H, Uto T, Bando M, et al. Interstitial pneumonia with anti-PL-7 antibody difficult to distinguish from rheumatoid lung. Nihon Kokyuki Gakkai Zasshi 2011;49:780-5. [PubMed]
- Park CK, Kim TJ, Cho YN, et al. Development of antisynthetase syndrome in a patient with rheumatoid arthritis. Rheumatol Int 2011;31:529-32. [Crossref] [PubMed]
- Watanabe K, Handa T, Tanizawa K, et al. Detection of antisynthetase syndrome in patients with idiopathic interstitial pneumonias. Respir Med 2011;105:1238-47. [Crossref] [PubMed]
- Tomioka H, Kaneko M, Kogata Y, et al. A case of interstitial lung disease with anti-EJ and anti-CCP antibodies preceding rheumatoid arthritis. Respir Investig 2012;50:66-9. [Crossref] [PubMed]
- Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement:idiopathic pulmonary fibrosis: idiopathic pulmonary fibrosis:evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788-824. [Crossref] [PubMed]
- Zamora AC, Hoskote SS, Abascal-Bolado B, et al. Clinical features and outcomes of interstitial lung disease in anti-Jo-1 positive antisynthetase syndrome. Respir Med 2016;118:39-45. [Crossref] [PubMed]
- Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733-48. [Crossref] [PubMed]
- Park WH, Kim SS, Shim SC, et al. Visual Assessment of chest computed tomography findings in anti-cyclic citrullinated peptide antibody positive rheumatoid arthritis: is it associated with airway abnormalities? Lung 2016;194:97-105. [Crossref] [PubMed]
- Fischer A, Swigris JJ, du Bois RM, et al. Anti-synthetase syndrome in ANA and anti-Jo-1 negative patients presenting with idiopathic interstitial pneumonia. Respir Med 2009;103:1719-24. [Crossref] [PubMed]
- Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246:697-722. [Crossref] [PubMed]
- Travis WD, Hunninghake G, King TE Jr, et al. Idiopathic nonspecific interstitial pneumonia: report of an American Thoracic Society project. Am J Respir Crit Care Med 2008;177:1338-47. [Crossref] [PubMed]
- Kondoh Y, Taniguchi H, Kitaichi M, et al. Acute exacerbation of interstitial pneumonia following surgical lung biopsy. Acute exacerbation of interstitial pneumonia following surgical lung biopsy. Respir Med 2006;100:1753-9. [Crossref] [PubMed]
- Tanaka N, Kim JS, Newell JD, et al. Rheumatoid arthritis-related lung diseases: CT findings. Radiology 2004;232:81-91. [Crossref] [PubMed]
- Park JH, Kim DS, Park IN, et al. Prognosis of fibrotic interstitial pneumonia: idiopathic versus collagen vascular disease-related subtypes. Am J Respir Crit Care Med 2007;175:705-11. [Crossref] [PubMed]
- Suda T. Up-to-date information on rheumatoid arthritis-associated interstitial lung disease. Clin Med Insights Circ Respir Pulm Med 2016;9:155-62. [PubMed]
- Sasano H, Hagiwara E, Kitamura H, et al. Long-term clinical course of anti-glycyl tRNA synthetase (anti-EJ) antibody-related interstitial lung disease pathologically proven by surgical lung biopsy. BMC Pulm Med 2016;16:168. [Crossref] [PubMed]
- Marie I, Dominique S, Janvresse A, et al. Rituximab therapy for refractory interstitial lung disease related to antisynthetase syndrome. Respir Med 2012;106:581-7. [Crossref] [PubMed]
- Meyer A, Lefevre G, Bierry G, et al. In antisynthetase syndrome, ACPA are associated with severe and erosive arthritis: an overlapping rheumatoid arthritis and antisynthetase syndrome. Medicine (Baltimore) 2015;94. [Crossref] [PubMed]
- Yamakawa H, Hagiwara E, Kitamura H, et al. Predictive Factors for the Long-Term Deterioration of Pulmonary Function in Interstitial Lung Disease Associated with Anti-Aminoacyl-tRNA Synthetase Antibodies. Respiration 2018;96:210-21. [PubMed]
- Ishiguro T, Takayanagi N, Miyahara Y, et al. Antisynthetase (anti PL-7 antibody) syndrome presenting as a skin rash and exacerbation of interstitial pneumonia during treatment for rheumatoid arthritis. Nihon Kokyuki Gakkai Zasshi 2010;48:240-6. [PubMed]


