
Do toxicity patterns vary between programmed death-1 and programmed death ligand-1 inhibitors?
There are currently four immune checkpoint inhibitors (ICI) approved for the treatment of stage III or stage IV non-small cell lung cancer (NSCLC) in the United States, with dozens more in clinical development. Nivolumab and pembrolizumab are programmed death-1 (PD-1) antibodies, while atezolizumab and durvalumab are antibodies to programmed death ligand-1 (PD-L1) (1-5). Since the initial approval of checkpoint inhibitors in 2015 for second-line treatment of metastatic NSCLC, these agents have transformed the management of locally advanced and metastatic NSCLC. ICIs are associated with durable responses for select patients and tumor expression of PD-L1 and tumor mutational burden have been utilized as predictive biomarkers (6-8). Overall these therapies tend to be better tolerated than cytotoxic chemotherapy, yet are associated with a unique set of immune-mediated toxicities which can be serious and sometimes fatal (9,10). Thus far no reliable predictor for these immune-related adverse events (irAE) has been discovered, and their identification and treatment remain an area of active research (11). In this context, the study by Pillai and colleagues to evaluate differences in toxicity between PD-1 and PD-L1 inhibitors adds to our evolving understanding of these powerful therapies when given as monotherapy (12).
Pillai and colleagues conducted a systemic review of trials performed between 2000–2016, including publications as well as clinical abstracts from annual meetings of relevant organizations. The final analysis included 23 trials of PD-1 (n=12) and PD-L1 (n=11) inhibitor monotherapy in patients with NSCLC after excluding 526 entries that were case reports, reviews, or did not contain toxicity data. The PD-1 inhibitors included nivolumab and pembrolizumab, and PD-L1 inhibitors included atezolizumab, durvalumab, and avelumab. Of note, avelumab is not currently one of the four Food and Drug Administration (FDA)-approved ICI options in NSCLC. In total, these trials included 5,744 patients, with more patients included from PD-1 trials (n=3,284) compared to PD-L1 trials (n=2,460). The analysis included trials ranging from the large CheckMate 153 study of 824 patients receiving nivolumab in community oncology practices to much smaller trials of only a few dozen patients. The trials took place largely in North America and Europe. The two groups were well matched in terms of age, gender, smoking history, and performance status, although there were slightly more patients with squamous cell histology in the PD-L1 trials compared to PD-1 trials (32% vs. 25%, P=0.6). The vast majority of patients were treated in the second line of therapy or beyond. However, three of the studies of nivolumab (n=337) and one study of durvalumab (n=59) were performed in the front-line setting. For the three pembrolizumab trials included, the doses used were 10 mg/kg in one trial (n=18) and a mix of doses (2 and 10 mg/kg) in the other two trials (n=1,185).
The overall incidence of any toxicity was similar between the PD-1 and PD-L1 inhibitor trials (64% vs. 66% respectively, P=0.80), and there was no significant difference in the rate of serious adverse events (AE’s) (grade 3–4 AE, 13% vs. 21%, P=0.15). There was also no significant difference between the rate of irAE overall between the PD-1 and PD-L1 trials (16% vs. 11%, respectively, P=0.07) or of serious irAE (3% vs. 5%, P=0.40). Pneumonitis of any grade occurred more frequently in PD-1 trials (4% vs. 2%, P=0.01). All 5 drugs were associated with overall AE rates between 62% (nivolumab) and 75% (durvalumab), however it should be noted that only 442 (18% of patient included from PD-L1 trials, and 8% of patients overall) were treated with durvalumab, and one of the three durvalumab studies occurred in the first line setting. The overall response rate was similar between the two groups (19% in PD-1 and 18.6% in PD-L1 trials, P=0.17)
The similar AE and irAE overall between the trials of PD-1 and PD-L1 inhibitors is reassuring. The increased rate of pneumonitis reported in PD-1 trials deserves further consideration. A prior retrospective study of 915 patients demonstrated an increased risk for pneumonitis in patients receiving combination immunotherapy [PD-1/L1 with cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4)], but there was no significant difference in risk between patients treated with anti-PD-1 vs. anti-PD-L1 therapies (13). However, this study included patients with multiple cancers in addition to NSCLC, and the rate of pneumonitis was numerically higher in PD-1 treated patients [22 out of 564 (4%) vs. 2 out of 152 (1%), P=0.13], although this did not meet statistical significance. A prior meta-analysis of 19 trials demonstrated an increased rate of pneumonitis in patients treated with PD-1 compared to PD-L1 therapies (3.6% vs. 1.3%, P=0.001) (14). This study included many of the same trials that were analyzed as part of the present study, including both publications and clinical abstracts. As Pillai et al. point out, nearly all of the trials of PD-1 inhibitors included in the analysis were published studies with only one abstract included out of the 12 PD-1 trials. Interestingly, this abstract was from the CheckMate 153 trial, and data have since been published on this study which included a total of 1,420 patients compared to 824 in the abstract (15). This trial is notable because it included two dose regimens of nivolumab given as either a 30- or 60-minute intravenous infusion every two weeks. Although the two infusion strategies appear to result in similar outcomes, the lack of availability of patient-level data precludes deeper analysis for confounding variables. By comparison, 10 out of the 11 PD-L1 trials in the meta-analysis were presented as abstracts at conference meetings. The difference in toxicity may be due to the fact that the data from presentations are not as mature as that available in a publication, which the authors point out. The fast pace of oncology trials has led to an increasing reliance on conferences as a means for sponsors to deliver “late-breaking” data. A recent study showed that 31% of clinical trials in lung cancer are presented multiple times in abstract form before final publication (16). As each presentation may focus on a different aspect of the study—for instance, subpopulations or biomarker endpoints—the data included in these presentations are generally not as complete as in the publication. Therefore, the increased rate in pneumonitis in patients treated with PD-1 inhibitors seen in this study may be impacted by the less rigorous data collection and reporting that is often encountered in meeting presentations compared to publications in high-impact, rigorously peer-reviewed journals.
The current study should be placed into context of the rapidly evolving treatment landscape for NSCLC. Treatment with ICI is now being offered as consolidation after chemoradiation in stage III NSCLC (1), and combination chemotherapy-ICI is being utilized in the first line setting for metastatic disease (17). Importantly, both of these approvals were based on trials that demonstrated an improvement in overall survival, the gold standard endpoint in cancer clinical trials, although at the time of publication only data on progression-free survival was available in the Pacific study. As more patients are treated with immunotherapy in novel combinations and sequences of treatment, it will be vital to evaluate patterns and predictors of irAE independently for each scenario. For instance, although these data appear to show an increased risk for pneumonitis in patients treated with PD-1 inhibitors, the context of treatment must be accounted for as well as the specific therapy. The Pacific study demonstrated a rate of pneumonitis of 33.9% overall (n=161) in patients treated with durvalumab compared to 24.8% for patients treated with placebo, including 3.4% grade 3 or 4 pneumonitis (1). Given that the patient population in this study recently completed radiation therapy, some of these toxicities are likely related to or at least exacerbated by radiation, which is key to critically interpreting the data. The assessment of toxicity in future clinical trials of ICI (both PD-1 and PDL-1 antibodies) in combination with chemoradiation in unresectable stage 3 disease may be complicated by the temporal placement of the checkpoint inhibitor (prior to chemoradiation, concurrent ICI with chemoradiation, and/or consolidation therapy after chemoradiation). In the KEYNOTE-189 trial of pembrolizumab with chemotherapy, the rate of pneumonitis was 4.4% (n=18) in 405 patients compared to 2.5% (n=5) in patients treated with chemotherapy plus placebo, although the rate of serious (grade 3–5) pneumonitis was similar between the two groups: 2.7% (n=11) in the pembrolizumab treated patients compared to 2.0% (n=4) in the chemotherapy plus placebo group. In the IMpower 150 trial, the rate of pneumonitis was 2.8% (1.5% grade 3 or 4) for patients treated with atezolizumab, bevacizumab, carboplatin, and paclitaxel, compared to 1.3% (0.5% grade 3 or 4) for those treated without atezolizumab (18). Overall, although combination therapy with ICI certainly leads to an improvement in outcomes for some patients, there appears to be a trade off in terms of toxicities including serious and fatal events (Table 1). The rate of AE leading to death in KEYNOTE-189 was 6.7% in the pembrolizumab plus chemotherapy arm (27/405 patients) (17), which is considerably higher than previously reported for platinum and pemetrexed combination therapy (19). The nature of toxicities that occur during combination therapy is also unpredictable, such as the higher incidence of febrile neutropenia and acute kidney injury in patients treated with pembrolizumab-chemotherapy compared to chemotherapy-placebo in KEYNOTE-189 (17). There are currently no known clinical or laboratory biomarkers to predict these serious toxicities, which are urgently needed to aid in clinical decision making.
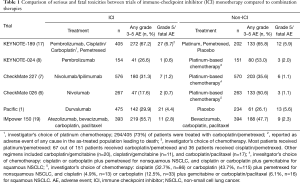
Full table
Given the multiple new emerging treatment options for patients with advanced NSCLC, studies like the one performed by Pillai and colleagues serve the important role of critically evaluating a growing body of literature where data is rapidly disseminated. Any differential toxicities of ICI themselves when given as monotherapy and as part of novel combinations (chemotherapy, radiation, other immune modulators) must be fully evaluated in order to best inform our clinical decision making, to guide our patients in their own decisions, and to raise awareness of patterns and symptoms that might be signs of unique or uncommon toxicities.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the Treatment of Non–Small-Cell Lung Cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Carbone DP, Reck M, Paz-Ares L, et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med 2017;376:2415-26. [Crossref] [PubMed]
- Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N Engl J Med 2018;378:2093-104. [Crossref] [PubMed]
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med 2018;378:158-68. [Crossref] [PubMed]
- Nishino M, Giobbie-Hurder A, Hatabu H, et al. Incidence of Programmed Cell Death 1 Inhibitor-Related Pneumonitis in Patients With Advanced Cancer: A Systematic Review and Meta-analysis. JAMA Oncol 2016;2:1607-16. [Crossref] [PubMed]
- Weber JS, Postow M, Lao CD, et al. Management of Adverse Events Following Treatment With Anti-Programmed Death-1 Agents. Oncologist 2016;21:1230-40. [Crossref] [PubMed]
- Pillai RN, Behera M, Owonikoko TK, et al. Comparison of the toxicity profile of PD-1 versus PD-L1 inhibitors in non-small cell lung cancer: A systematic analysis of the literature. Cancer 2018;124:271-7. [Crossref] [PubMed]
- Naidoo J, Wang X, Woo KM, et al. Pneumonitis in Patients Treated With Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. J Clin Oncol 2017;35:709-17. [Crossref] [PubMed]
- Khunger M, Rakshit S, Pasupuleti V, et al. Incidence of Pneumonitis With Use of Programmed Death 1 and Programmed Death-Ligand 1 Inhibitors in Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis of Trials. Chest 2017;152:271-81. [Crossref] [PubMed]
- Waterhouse D, Horn L, Reynolds C, et al. Safety profile of nivolumab administered as 30-min infusion: analysis of data from CheckMate 153. Cancer Chemother Pharmacol 2018;81:679-86. [Crossref] [PubMed]
- Ellis-Caleo T, Lisberg A, Tucker DA, et al. High-profile studies frequently and repetitively present data on the same patients, particularly in immunotherapy studies. J Thorac Dis 2018;10:S397-403. [Crossref] [PubMed]
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non–Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. [Crossref] [PubMed]
- Scagliotti GV, Parikh P. Phase III Study Comparing Cisplatin Plus Gemcitabine With Cisplatin Plus Pemetrexed in Chemotherapy-Naive Patients With Advanced-Stage Non–Small-Cell Lung Cancer. J Clin Oncol 2008;26:3543-51. [Crossref] [PubMed]


