Surgical management of non-cystic fibrosis bronchiectasis
Introduction
Surgical treatment for bronchiectasis has a history that spans over a century and was first reported by Heidenhain (1). Resection surgery served as the main treatment for this disease until the 1950s when modern antibiotics and immunization became available (2-4). Owing to the better control of tuberculosis, effective antibiotics, routine immunization, and improvement in social conditions, bronchiectasis was considered an “orphan disease” in the late 1980s (5). By the late 1970s, lung cancer, instead of infectious diseases such as tuberculosis and bronchiectasis, had become the main candidate for lung resection and surgery for infectious disease became relatively uncommon for thoracic surgeons. However, the global incidence and prevalence of bronchiectasis in adults has steadily increased (6-8). Possible reasons for this increase are frequent radiological detection due to the greater use of computed tomography (CT) and the more frequent presence of immunosuppressed states (8-10). Moreover, the prevalence of nontuberculous mycobacterial (NTM) lung disease has increased (11-13), especially the nodular/bronchiectatic type, affecting mainly the middle lobe and lingular segments. Thus, with the resurgent global interest in bronchiectasis and with newer knowledge of the condition as disclosed by modern techniques, the role of surgery in the management of bronchiectasis also requires reexamination.
Indication and rationale
The most widely known model of the development of bronchiectasis is Cole’s “vicious cycle hypothesis” (14). According to this model, the pathophysiology of bronchiectasis is commonly described to comprise four distinct phases: “chronic infection”, “chronic inflammatory response”, “impaired lung defense to microorganisms/impaired mucociliary function”, and “structural change of the lung”. Depending on the etiology in each patient, each of the four phases can be the entry point and can trigger the cycle of bronchiectasis development [Figure 1 (15)].
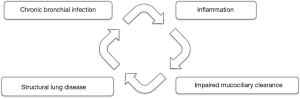
Post-infectious bronchiectasis and other structural lung diseases
Surgery is most efficient for symptomatic bronchiectasis whose vicious cycle is triggered by the localized structural change of the airway in otherwise healthy individuals. Typically, the causes of the airway injury are childhood infection, such as tuberculosis, measles, pertussis, etc.; post-infectious bronchiectasis. Endobronchial obstruction with a tumor or foreign body and extraluminal compression by peribronchial pathology such as lymphadenopathy (e.g., middle lobe syndrome) also cause airway distortion and can induce peripheral bronchiectasis. Theoretically, antimicrobial therapy penetrates poorly into sites where severe parenchymal distortion and destruction are present in Figure 2. Thus, these sites act as microbial reservoirs to induce clinical symptoms and to seed new infection to other parts of the lung. In addition, the bronchiectatic diseased lung that harbors inflammation and infection does not contribute to ventilation. In cases with relatively localized diseases that cause frequent exacerbations and sputum expectorations regardless of optimal medical management, surgery can greatly contribute to significant symptom relief and prevent the infectious contamination of adjacent lung parenchyma (15-30). In other words, poor response to medical treatment allows examination for the need of surgery. In such a setting, the complete resection of a damaged part of the lung is ideal for achieving better surgical outcomes (16,17,19-23,25-29). In our previous study (27), we examined the clinical difference between the types of postoperative residual regions. Via multivariate analysis, the residual bronchiectatic regions had an adverse impact on the surgical outcome, whereas, the residual nodular infiltrative regions did not. This suggested that, as the residual nodular region can be well controlled medically, this type of region does not need to be removed. Medical management is especially difficult when the targeted microorganism is refractory or resistant to antimicrobial drugs, such as Aspergillus, multidrug-resistant tuberculosis, or MRSA, justifying surgery earlier if localized. Persistent Pseudomonas aeruginosa infection is also known to be a risk factor for earlier lung function decline and more frequent exacerbations, which can be explained by airway damage induced by the numerous substances produced by Pseudomonas aeruginosa (31,32). Patients with massive hemoptysis also greatly benefit from surgery.
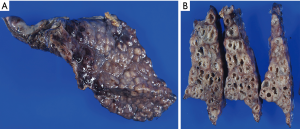
Destroyed lung
“Destroyed lung” is a large destruction of the lung as a sequela of severe and destructive infectious disease, predominantly comprising tuberculosis and pulmonary abscess. Occasionally, it occurs in the residual lobe after lobectomy on the ipsilateral side (33). This is the most aggressive and extended form of structural localized damage of the airway. For this severe presentation, surgery is inevitable for cure; however, it is associated with a high morbidity and mortality, especially if completion pneumonectomy is required (34,35).
NTM lung disease
It is generally accepted that NTM is a pathogen that can directly succeed or even precede bronchiectasis development, representing the “chicken or the egg” dilemma (36-38). NTM lung disease is divided into two types radiologically, the fibrocavitary and nodular/bronchiectatic types (39), and the latter is particularly difficult to discern, clinically or radiologically, from post-infectious bronchiectasis colonized or infected with NTM.
Recently, the prevalence of NTM lung disease has been increasing, owing especially to the increase of the nodular/bronchiectatic subtype; typically presenting as bronchiectatic middle-lobe and lingular segments in middle-aged slender, non-smoking women (11,13,40-42). Consequently, in the surgical reports of NTM lung disease published recently, a predominance of women with bronchiectatic disease has been observed (43-45).
From the opposite perspective, according to the US bronchiectasis research registry, up to 63% of bronchiectasis cases were related to NTM infection (46). In our previous report on the outcome of surgical treatment for bronchiectasis, 67.7% of the cases were associated with NTM infection (27). However, the frequency of NTM infection among bronchiectasis patients largely differs between regions. Table 1 presents a summary of studies on the surgical treatment of bronchiectasis and shows that the microorganisms identified differed, reflecting geographical diversity (16-30).
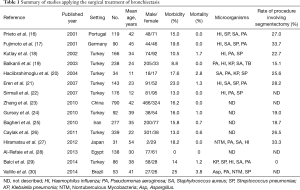
Full table
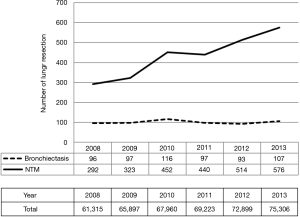
According to surveys of thoracic surgery by the Japanese Association for Thoracic Surgery, the number of surgeries for NTM lung disease is increasing in Japan, whereas, that of bronchiectasis has remained the same (Figure 3) (47-51). However, as it is obvious that the increase of NTM is mainly due to the nodular/bronchiectatic type, the total number of surgeries has been increasing for these two inextricably linked diseases with bronchiectatic appearance.
In cases whose bronchiectatic NTM lung disease is regarded to result from NTM infection or colonization in the focal damaged lung, the treatment strategy is the same as for post-infectious bronchiectasis. However, in a large portion of NTM patients with bronchiectatic regions, the pathogenesis seems to be mainly due to systemic problems. In other words, the initial phase of the vicious cycle may not be triggered by the “structural change of the lung” but by another unknown etiology; idiopathic bronchiectasis with NTM infection. Recent reports of the association between certain genetic changes and the characteristic body habitus or clinical presentation among nodular/bronchiectatic NTM patients provide some insight into this situation (52-54).
From the clinical perspective, the risk factors associated with the rapidness of lung function decline are reported to differ between bronchiectasis and NTM; in bronchiectasis, chronic Pseudomonas aeruginosa infections, frequent exacerbations, and systemic inflammation, and in NTM, young age, male sex, and a high radiographic score are associated risk factors apart from cavitary disease (55,56).
In NTM patients, quality of life is related to lung function such as forced vital capacity; FVC and diffusing capacity of the lung for carbon monoxide; DLCo. However, quality of life is related to the number of exacerbations and the amount of expectorated sputum in bronchiectasis patients but not to lung function or disease extent (57-59). The prevalence of Aspergillus-related lung disease is also higher among bronchiectatic NTM lung disease than among bronchiectasis without NTM infection (60).
As the pathophysiology and clinical behavior of these two groups should be viewed separately as noted above, the indications and rationale for surgery should also be considered differently. Mitchell proposed three main rationales for surgery in managing NTM lung disease: (I) to induce treatment success (future termination of drug therapy by extirpating the infected site), (II) to relieve symptoms such as hemoptysis, and (III) to limit or to slow down the disease progression, in which the goal of surgery is not complete eradication but alleviation of the infection (61). The third rationale is particularly indicated for cases with extended NTM lung disease, on the basis of its difficulty in managing medically even with a multidrug regimen. The situation is more difficult in cases with Mycobacterium abscessus, which is notorious for its aggressive nature, macrolide-resistant Mycobacterium avium complex species, or Aspergillus infection. In such settings, the extent of the disease in terms of whether it is “focal” or “localized” is not necessarily an essential condition for surgery, which is based on the concept that the alleviation, and not extirpation, of the main gross irreversible parenchymal damage can occasionally be beneficial in selected cases, and can be associated with better subsequent management of the disease. More study and further evaluation of this concept is urgently awaited.
Usually, NTM lung disease takes a relatively silent and indolent course from focal to diffuse lung parenchymal destruction; however, there are some patients whose disease progresses rapidly. As long as NTM lung infection is regarded to result from a systemic problem that somehow triggers the “vicious cycle”, but not by the “structural change of the lung”, the postoperative burden should be minimized as much as possible.
To select appropriate surgical candidates at high-risk for progression in the early stage of NTM lung disease, it is crucial to identify patient predisposing factors (52-54) and NTM species with aggressive phenotypes or genes related to macrolide resistance (62-64).
As the context and reasons for removing the lung parenchyma with bronchiectasis vary as described above, it is essential to perform a drastic host defense survey according to established priorities and to apply a multidisciplinary approach to assess and manage the disease holistically.
Preoperative management
Before the advent of antibiotics, the operative outcome of bronchiectasis was associated with a high incidence of bronchopleural fistula (BPF), empyema, and perioperative mortality of as high as 46% (2,3). Thus, operative success depends on accurate perioperative medical management. Smears and cultures of sputum or bronchial washing should be examined in all patients and antibiotic administration should be initiated 1 to 2 weeks preoperatively, referring susceptibility testing to produce a bacterial nadir at the time of surgery. Antifungal agents or multidrug regimens for Mycobacterium including intravenous aminoglycoside should be administered for more than 3 months preoperatively (65). Preoperative bronchoscopy is mandatory in all cases. The aim of this is to identify causative microorganisms and to rule out endobronchial pathology that may cause peripheral bronchiectasis.
Concomitant diseases such as diabetes mellitus, anemia, and malnutrition should be strictly monitored and controlled. The influence of immunosuppressants, low-dose steroid, or cytotoxic chemotherapy should be carefully assessed and minimized.
All patients should undergo contrast-enhanced multidetector row CT (MDCT) preoperatively to assess the extent of the diseased lung to remove in association with lobar fissure and pulmonary vessels. In particular, three-dimensional (3D) CT angiography with contrast enhancement allows more detailed information of the anatomic variation of the pulmonary vessels (66,67) that occasionally make lung resection hazardous, especially when the interlobar fissure is incomplete and the hilar adhesion and scarring are severe because of chronic and recurrent inflammation. Moreover, sublobar resection (segmentectomy and subsegmentectomy) is frequently required for the surgical treatment of bronchiectasis because of the nature of the disease (4). The 3D visualization of a more detailed anatomy of the peripheral lung unit and the distribution of the disease in association with intersegmental veins is very useful and ensures safety in such instances (Figure 4). Moreover, preoperative MDCT angiography is useful for detecting the development of the systemic blood circulation, bronchial or parietal, with reverse blood circulation from the systemo-pulmonary anastomoses, which may cause hemoptysis and can be a source of major operative blood loss. Based on this, the application of preoperative bronchial arterial embolization should be assessed and discussed with the interventional radiologist (68).
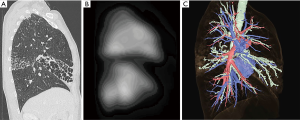
In addition to radiological assessment, other standard preoperative workups including pulmonary function tests, arterial blood gas analysis, and pulmonary ventilation/perfusion (V/Q) scan with the occasional use of cardiopulmonary exercise testing should be performed to select patients with adequate pulmonary reserve, who are eligible for anatomic lung resection. In most cases, pulmonary V/Q scan depicts the perfusion defect in the diseased lung, which is beyond the access of medical treatment agents via the bloodstream, and shows that the surgery would remove the non-functional lung (Figure 4). Cardiac evaluation involves echocardiography to ascertain possible valvulopathy and the presence of pulmonary hypertension.
Surgical technique
Surgery should be performed under general anesthesia. Early lung isolation using a double-lumen endobronchial tube facilitates the prevention of the spillage or secretions from the affected side during surgery. A local injection of anesthetic into the thoracic epidural or paravertebral space is recommended for intra- and postoperative analgesia. Bronchoscopy immediately before the initiation of the surgery is mandatory to clear the airway to the diseased lung of fetid secretions. Additionally, evaluating the intraluminal inflammation, including submucosal granuloma, to avoid involving it in the bronchial sealing ensures bronchial stump safety. When segmentectomy or subsegmentectomy is planned, endobronchial recognition and the confirmation of corresponding bronchi are mandatory.
Pulmonary resection is achieved through a lateral thoracotomy. The most commonly performed procedure is lobectomy; however, segmentectomy or lobectomy with segmentectomy is frequently required (Table 1) because bronchiectasis namely extends along bronchial branching. Historically, segmental pulmonary resection was introduced as a novel technique in the late 1940s and was initially devised as an ideal procedure for bronchiectasis, being regarded as “segmental pneumonectomy” (4). Interlobar fissure with adhesion, incomplete interlobar fissure, and the intersegmental plane are divided with electrocautery or stapling. Either way, determining the resection line by manipulating the diseased area with bronchiectasis in order to extirpate it is very important. The bronchus is usually divided and closed with staples and is reinforced with muscle flaps in patients at high risk for BPF, such as in those with positive preoperative sputum or diabetes mellitus and in those who require pneumonectomy.
Owing to the advent of thoracoscopy, adhesions, especially in the apex and back side of the thoracic wall beneath the thoracic incision, are visualized well and, thus, the size of skin incision can be occasionally minimized. However, adhesion and symphysis are often very dense in cases s with preoperative frequent and repeated exacerbations. For bronchiectasis with massive adhesion throughout the thoracic cavity, skin incision should be extended so that the access and taping of the pulmonary vessels at the hilum can be arranged easily, even with intrapericardial manipulation, in order to ensure safety. In such occasions, postoperative excessive negative pressure in the residual space and the intraoperative pleural soilage are risk factors for the development of significant postoperative morbidities such as BPF and empyema. Therefore, to minimize the size of the residual space and to avoid polluting it are keys to overcoming postoperative morbidity. Care should be taken to avoid entry into the infected lung parenchyma so that fetid and highly virulent infection would not remain within the thoracic space. After the removal of the diseased lung, it is needful to fully expand the residual healthy lung by mobilizing it with minimal damage. Extensive extrapleural dissection together with, if needed, two or three rib resections is an effective technique for lessening the negative pressure of the residual space; i.e., the effect of thoracoplasty. Latissimus dorsi muscle flap for buttressing the bronchial stump is often applied in cases with surrounding gross and severe adhesion. The purpose of this maneuver is to protect the intrathoracic space from bronchial stump complications, to cover the residual lung surface, and to minimize the residual space. Moreover, after harvesting the latissimus dorsi muscle flap, a supplementary incision of the rib cage via the seventh or eighth intercostal space without an extra skin incision or its extension becomes available, which is useful in the dissection and mobilization of severe adhesion in the lower part of the thoracic space.
Abundant water irrigation into the thoracic space and sending the sample of that lavage fluid to bacteriological testing is necessary. All resected specimens are to be sent for pathologic and bacteriologic examination. Aggressive chest physiotherapy and nutritional supplementation in the postoperative period are also important.
Surgical outcome
No randomized controlled trials of surgical treatment versus standard care for bronchiectasis have been reported. In a recent meta-analysis by Fan et al., three main outcomes: mortality, morbidity (adverse event), and quality of life improvement after surgery for bronchiectasis were assessed among 4,614 patients from 35 studies (69). The pooled mortality was 1.5%, while the morbidity was 16.7%. The most common complications after surgery included atelectasis requiring therapeutic bronchoscopy, prolonged air leakage (>7 days), wound infection, cardiac arrhythmias, and empyema. Symptom improvement after surgery was noted in 95% (66.5% became asymptomatic), while no clinical improvement was observed in 9.1% of patients. Table 1 shows the detailed data of patients’ background and surgical outcome from representative reported studies, suggesting that although there is a great regional difference in patients’ background and causative microorganisms, surgery is effective in a selected population (16-30). The key to a favorable outcome is the complete resection of the bronchiectasis while leaving an adequate respiratory reserve (16,17,19-23,25,29).
Vallilo et al. demonstrated that surgery for bronchiectasis significantly improved the quality of life and exercise capacity irrespective of a slight decline in lung volume at the 9-month follow-up (30). In patients with bronchiectasis, quality of life is not necessarily associated with lung function and disease extent on CT but with the number of exacerbations and amount of daily sputum. Moreover, the diseased lung with bronchiectasis does not contribute to ventilation. Through these, Vallilo et al. emphasized the importance of assessing the postoperative quality of life improvement among refractory and symptomatic patients in order to evaluate the merit of surgery and not just the rate of symptom control, morbidity, or lung function.
Conclusions
The pathophysiology and the extent of inflammation notably differ in each case as shown in this review because the term “bronchiectasis” is just an expression of the appearance of a lung as a result of certain episodes and their unknown influence. The key to a favorable surgical outcome is a multidisciplinary approach starting from the appropriate selection of the surgical candidate, with optimal preoperative management and suitable surgical technique at the right timing, and followed by appropriate postoperative support.
Acknowledgements
We thank Dr. Atsuyuki Kurashima, Dr. Yuka Sasaki, Dr. Kozo Morimoto and Dr. Hitoshi Takeuchi for their advice. We also thank Dr. Kiyomi Shimoda, Dr. Takayuki Nakagawa, Dr. Takeo Togo, Dr. Jun Atsumi and Dr. Tsutomu Yoshida for their support in clinical managements.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Heidenhain L. Verhandlungen der Deutschen Gesellschaft fur Chirurgie. Berlin: Tagung. Verlagsort 1901;30:463.
- Lindskog GE, Hubbel DS. An analysis of 251 cases of bronchiectasis. Surg Gynecol Obstet 1955;100:643-50. [PubMed]
- Ochsner A, DeBakey M, Decamp PT. Bronchiectasis; its curative treatment by pulmonary resection; an analysis of 96 cases. Surgery 1949;25:518-32. [PubMed]
- Overholt RH, Langer L. A new technique for pulmonary segmental resection; its application in the treatment of bronchiectasis. Surg Gynecol Obstet 1947;84:257-68. [PubMed]
- Barker AF, Bardana EJ. Bronchiectasis: update of an orphan disease. Am Rev Respir Dis 1988;137:969-78. [Crossref] [PubMed]
- Ringshausen FC, de Roux A, Pletz MW, et al. Bronchiectasis-associated hospitalizations in Germany, 2005-2011: a population-based study of disease burden and trends. PLoS One 2013;8. [Crossref] [PubMed]
- Seitz AE, Olivier KN, Steiner CA, et al. Trends and burden of bronchiectasis-associated hospitalizations in the United States, 1993-2006. Chest 2010;138:944-9. [Crossref] [PubMed]
- Quint JK, Millett ER, Joshi M, et al. Changes in the incidence, prevalence and mortality of bronchiectasis in the UK from 2004 to 2013: a population-based cohort study. Eur Respir J 2016;47:186-93. [Crossref] [PubMed]
- Dodd JD, Souza CA, Muller NL. Conventional high-resolution CT versus helical high-resolution MDCT in the detection of bronchiectasis. AJR Am J Roentgenol 2006;187:414-20. [Crossref] [PubMed]
- Goeminne PC, De Soyza A. Bronchiectasis: how to be an orphan with many parents? Eur Respir J 2016;47:10-3. [Crossref] [PubMed]
- Kendall BA, Winthrop KL. Update on the epidemiology of pulmonary nontuberculous mycobacterial infections. Semin Respir Crit Care Med 2013;34:87-94. [Crossref] [PubMed]
- Donohue MJ, Wymer L. Increasing prevalence rate of nontuberculous mycobacteria infections in five states, 2008-2013. Ann Am Thorac Soc 2016;13:2143-50. [Crossref] [PubMed]
- Morimoto K, Iwai K, Uchimura K, et al. A steady increase in nontuberculous mycobacteriosis mortality and estimated prevalence in Japan. Ann Am Thorac Soc 2014;11:1-8. [Crossref] [PubMed]
- Cole PJ. Inflammation: a two-edged sword - the model of bronchiectasis. Eur J Respir Dis Suppl. 1986;147:6-15. [PubMed]
- Polverino E, Goeminne PC, McDonnell MJ, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J 2017;50. [Crossref] [PubMed]
- Prieto D, Bernardo J, Matos MJ, et al. Surgery for bronchiectasis. Eur J Cardiothorac Surg 2001;20:19-23, discussion 23-4. [Crossref] [PubMed]
- Fujimoto T, Hillejan L, Stamatis G. Current strategy for surgical management of bronchiectasis. Ann Thorac Surg 2001;72:1711-5. [Crossref] [PubMed]
- Kutlay H, Cangir AK, Enön S, et al. Surgical treatment in bronchiectasis: analysis of 166 patients. Eur J Cardiothorac Surg 2002;21:634-7. [Crossref] [PubMed]
- Balkanli K, Genc O, Dakak M, et al. Surgical management of bronchiectasis: analysis and short-term results in 238 patients. Eur J Cardiothorac Surg 2003;24:699-702. [Crossref] [PubMed]
- Haciibrahimoglu G, Fazlioglu M, Olcmen A, et al. Surgical management of childhood bronchiectasis due to infectious disease. J Thorac Cardiovasc Surg 2004;127:1361-5. [Crossref] [PubMed]
- Eren S, Esme H, Avci A, et al. Risk factors affecting outcome and morbidity in the surgical management of bronchiectasis. J Thorac Cardiovasc Surg 2007;134:392-8. [Crossref] [PubMed]
- Sirmali M, Karasu S, Turut H, et al. Surgical management of bronchiectasis in childhood. Eur J Cardiothorac Surg 2007;31:120-3. [Crossref] [PubMed]
- Zhang P, Jiang G, Ding J, et al. Surgical treatment of bronchiectasis: A retrospective analysis of 790 patients. Ann Thorac Surg 2010;90:246-50. [Crossref] [PubMed]
- Gursoy S, Ozturk AA, Ucvet A, et al. Surgical management of bronchiectasis: the indications and outcomes. Surg Today 2010;40:26-30. [Crossref] [PubMed]
- Bagheri R, Haghi SZ, Fattahi Masoum SH, et al. Surgical management of bronchiectasis: analysis of 277 patients. Thorac Cardiovasc Surg 2010;58:291-4. [Crossref] [PubMed]
- Caylak H, Genc O, Kavakli K, et al. Surgical management of bronchiectasis: a collective review of 339 patients with long-term follow-up. Thorac Cardiovasc Surg 2011;59:479-83. [Crossref] [PubMed]
- Hiramatsu M, Shiraishi Y, Nakajima Y, et al. Risk factors that affect the surgical outcome in the management of focal bronchiectasis in a developed country. Ann Thorac Surg 2012;93:245-50. [Crossref] [PubMed]
- Al-Refaie RE, Amer S, El-Shabrawy M. Surgical treatment of bronchiectasis: a retrospective observational study of 138 patients. J Thorac Dis 2013;5:228-33. [PubMed]
- Balci AE, Balci TA, Ozyurtan MO. Current surgical therapy for bronchiectasis: surgical results and predictive factors in 86 patients. Ann Thorac Surg 2014;97:211-7. [Crossref] [PubMed]
- Vallilo CC, Terra RM, de Albuquerque AL, et al. Lung resection improves the quality of life of patients with symptomatic bronchiectasis. Ann Thorac Surg 2014;98:1034-41. [Crossref] [PubMed]
- Finch S, McDonnell MJ, Abo-Leyah H, et al. A comprehensive analysis of the impact of Pseudomonas aeruginosa colonization on prognosis in adult bronchiectasis. Ann Am Thorac Soc 2015;12:1602-11. [PubMed]
- Gellatly SL, Hancock RE. Pseudomonas aeruginosa: new insights into pathogenesis and host defenses. Pathog Dis 2013;67:159-73. [Crossref] [PubMed]
- Tanaka H, Matsumura A, Ohta M, et al. Late sequelae of lobectomy for primary lung cancer: fibrobullous changes in ipsilateral residual lobes. Eur J Cardiothorac Surg 2007;32:859-62. [Crossref] [PubMed]
- Carette MF, Blanchon F, Milleron B, et al. Destroyed lung. Sem Hop 1979;55:843-53. [PubMed]
- Sirmali M, Karasu S, Gezer S, et al. Completion pneumonectomy for bronchiectasis: morbidity, mortality and management. Thorac Cardiovasc Surg 2008;s56:221-5.
- Kim RD, Greenberg DE, Ehrmantraut ME, et al. Pulmonary nontuberculous mycobacterial disease: prospective study of a distinct preexisting syndrome. Am J Respir Crit Care Med 2008;178:1066-74. [Crossref] [PubMed]
- Fujita J, Ohtsuki Y, Shigeto E, et al. Pathological findings of bronchiectases caused by Mycobacterium avium intracellulare complex. Respir Med 2003;97:933-8. [Crossref] [PubMed]
- Griffith DE, Aksamit TR. Bronchiectasis and nontuberculous mycobacterial disease. Clin Chest Med 2012;33:283-95. [Crossref] [PubMed]
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007;175:367-416. [Crossref] [PubMed]
- Prevots DR, Marras TK. Epidemiology of human pulmonary infection with nontuberculous mycobacteria: a review. Clin Chest Med 2015;36:13-34. [Crossref] [PubMed]
- Namkoong H, Kurashima A, Morimoto K, et al. Epidemiology of pulmonary nontuberculous mycobacterial disease, Japan. Emerg Infect Dis 2016;22:1116-7. [Crossref] [PubMed]
- Kwak HJ, Moon JY, Choi YW, et al. High prevalence of bronchiectasis in adults: analysis of CT findings in a health screening program. Tohoku J Exp Med 2010;222:237-42. [Crossref] [PubMed]
- Watanabe M, Hasegawa N, Ishizaka A, et al. Early pulmonary resection for Mycobacterium avium complex lung disease treated with macrolides and quinolones. Ann Thorac Surg 2006;81:2026-30. [Crossref] [PubMed]
- Mitchell JD, Bishop A, Cafaro A, et al. Anatomic lung resection for nontuberculous mycobacterial disease. Ann Thorac Surg 2008;85:1887-92. [Crossref] [PubMed]
- Shiraishi Y, Katsuragi N, Kita H, et al. Adjuvant surgical treatment of nontuberculous mycobacterial lung disease. Ann Thorac Surg 2013;96:287-91. [Crossref] [PubMed]
- Aksamit TR, O’Donnell AE, Barker A, et al. Adult bronchiectasis patients: a first look at the US Bronchiectasis Research Registry. Chest 2017;151:982-92. [Crossref] [PubMed]
- Committee for Scientific Affairs. Thoracic and cardiovascular surgery in Japan during 2012: annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2014;62:734-64. [Crossref] [PubMed]
- Amano J, Kuwano H, Yokomise H. Thoracic and cardiovascular surgery in Japan during 2011: Annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2013;61:578-607. [Crossref] [PubMed]
- Kuwano H, Amano J, Yokomise H. Thoracic and cardiovascular surgery in Japan during 2010: annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2012;60:680-708. [Crossref] [PubMed]
- Committee for Scientific Affairs, Sakata R, Fujii Y, et al. Thoracic and cardiovascular surgery in Japan during 2009: annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2011;59:636-67. [Crossref] [PubMed]
- Sakata R, Fujii Y, Kuwano H. Thoracic and cardiovascular surgery in Japan during 2008: annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2010;58:356-83. [Crossref] [PubMed]
- Kim RD, Greenberg DE, Ehrmantrout ME, et al. Pulmonary Nontuberculous mycobacterial disease; prospective study of a distinct preexisting syndrome. Am J Respir Crit Care Med 2008;178:1066-74. [Crossref] [PubMed]
- Mai HN, Hijikata M, Inoue Y, et al. Pulmonary Mycobacterium avium complex infection associated with the IVS8T-5 allele of the CFTR gene. Int J Tuberc Lung Dis 2007;11:808-13. [PubMed]
- Bienvenu T, Sermet-Gaudelus I, Burgel PR, et al. Cystic fibrosis transmembrane conductance regulator channel dysfunction in non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med 2010;181:1078-84. [Crossref] [PubMed]
- Martínez-García MA, Soler-Cataluña JJ, Perpiñá-Tordera M, et al. Factors associated with lung function decline in adult patients with stable on-cystic fibrosis bronchiectasis. Chest 2007;132:1565-72. [Crossref] [PubMed]
- Lee MR, Yang CY, Chang KP, et al. Factors associated with lung function decline in patients with non-tuberculous mycobacterial pulmonary disease. PLoS One 2013;8. [Crossref] [PubMed]
- Girón Moreno RM, Fernandes Vasconcelos G, Cisneros C, et al. Presence of anxiety and depression in patients with bronchiectasis unrelated to cystic fibrosis. Arch Bronconeumol 2013;49:415-20. [Crossref] [PubMed]
- Olveira C, Olveira G, Gaspar I, et al. Depression and anxiety symptoms in bronchiectasis: associations with health-related quality of life. Qual Life Res 2013;22:597-605. [Crossref] [PubMed]
- Mehta M, Marras TK. Impaired health-related quality of life in pulmonary nontuberculous mycobacterial disease. Respir Med 2011;105:1718-25. [Crossref] [PubMed]
- Kunst H, Wickremasinghe M, Wells A, Wilson R. Nontuberculous mycobacterial disease and Aspergillus-related lung disease in bronchiectasis. Eur Respir J 2006;28:352-7. [Crossref] [PubMed]
- Mitchell JD. Surgical approach to pulmonary nontuberculous mycobacterial infections. Clin Chest Med 2015;36:117-22. [Crossref] [PubMed]
- Jeon K, Kwon OJ, Lee NY, et al. Antibiotic treatment of Mycobacterium abscessus lung disease: a retrospective analysis of 65 patients. Am J Respir Crit Care Med 2009;180:896-902. [Crossref] [PubMed]
- Harada T, Akiyama Y, Kurashima A, et al. Clinical and microbiological differences between Mycobacterium abscessus and Mycobacterium massiliense lung disease. J Clin Microbiol 2012;50:3556-61. [Crossref] [PubMed]
- Koh WJ, Jeon K, Lee NY, et al. Clinical significance of differentiation of Mycobacterium massiliense from Mycobacterium abscessus. Am J Respir Crit Care Med 2011;183:405-10. [Crossref] [PubMed]
- Nontuberculous Mycobacteriosis Control Committee of the Japanese Society for Tuberculosis; International Exchanging Committee of the Japanese Society for Tuberculosis. Guidelines for surgical therapy for pulmonary nontuberculous mycobacterial diseases. Kekkaku 2011;86:41-2. [PubMed]
- Watanabe S, Arai K, Watanabe T, et al. Use of three-dimensional computed tomographic angiography of pulmonary vessels for lung resections. Ann Thorac Surg 2003;75:388-92. [Crossref] [PubMed]
- Yamada S, Suga A, Inoue Y, et al. Use of multi-detector row angiography for the arrangement of video-assisted modified segmental resection. Eur J Cardiothorac Surg 2009;36:727-30. [Crossref] [PubMed]
- Sopko DR, Smith TP. Bronchial artery embolization for hemoptysis. Semin Intervent Radiol 2011;28:48-62. [Crossref] [PubMed]
- Fan LC, Liang S, Lu HW, et al. Efficiency and safety of surgical intervention to patients with non-cystic fibrosis bronchiectasis: a meta-analysis. Sci Rep 2015;5:17382. [Crossref] [PubMed]


