Medical management of bronchiectasis
Introduction
Bronchiectasis is a chronic lung disease caused by a cycle of infection and inflammation that results in permanent structural damage to the small airways and sometimes causes destruction of adjacent lung parenchyma (1). Originally described in 1819 by Laennec (2), the disease was thought to be of waning clinical significance in the 1970’s and 1980’s (3). However, the prevalence of the disease has resurged; in 2005 it was estimated that there were at least 100,000 individuals in the United States who had bronchiectasis unrelated to cystic fibrosis (4) and a subsequent epidemiologic study has shown a rising prevalence with an annual change of 8.74% per year (5). Bronchiectasis affects people across the spectrum of age but the highest prevalence is in women over the age of 60 years (5). Bronchiectasis is also common in other parts of the world; a recent publication from China reported that 1.2% of individuals over the age of 40 years have been diagnosed with the disease (6).
Bronchiectasis is a heterogeneous disease; severity is variable and is impacted by the extent of lung involvement, the microbiologic complications and co-existing disorders. Cystic fibrosis is a well-studied cause of bronchiectasis; this review will cover bronchiectasis unrelated to cystic fibrosis (i.e., non-cystic fibrosis bronchiectasis—“bronchiectasis” in this manuscript). Bronchiectasis may be caused by a variety of underlying conditions including genetic abnormalities, immunologic conditions, autoimmune diseases, obstructing airway lesions or chronic aspiration (7). It can also be related to pre-existing chronic obstructive pulmonary disease (COPD) or asthma. However, even with intensive investigation for an underlying cause, many cases are thought to be due to prior infections (with or without clear-cut documentation) or are considered to be idiopathic in origin (see Table 1). A common complication of bronchiectasis is infection; certain micro-organisms have a propensity for causing chronic infection in patients with bronchiectasis. In many cases, the infection occurs and becomes chronic because of the pre-existing lung disease but infections may also be the cause of the bronchiectasis, particularly non-tuberculous mycobacterial infections that cause nodular bronchiectasis (8). Patients with bronchiectasis are at risk for exacerbations; a recent consensus statement has defined bronchiectasis exacerbations (see Table 2) (9) and prevention of exacerbations is an important goal of treatment. Patients with frequent exacerbations are more likely to have decline in lung function and a worse prognosis compared to those who infrequently exacerbate (10). Two tools for assessing prognosis in bronchiectasis are the Bronchiectasis Severity Index (BSI) and the FACED score (11,12). Multi-morbidity is frequent in bronchiectasis; co-morbidities including malignancy, cognitive impairment, liver disease and cardiovascular disease have a negative impact on outcomes (13).

Full table
Medical management of patients with bronchiectasis include the following steps: (I) confirmation of the diagnosis; (II) evaluation for underlying disorders, particularly for treatable processes; (III) multi-modality approach to care, personalized to the individual patient.
Confirming the diagnosis of bronchiectasis
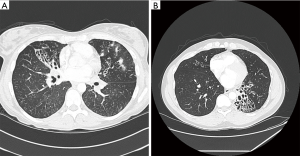
Patients with bronchiectasis present to health care providers with symptoms of chronic cough, chest congestion and sputum production; they generally report a history of frequent lower respiratory tract infections. Associated symptoms include wheezing, chest discomfort, hemoptysis, fever, malaise and fatigue. Though a routine chest radiograph can sometimes suggest the presence of bronchiectasis, a high resolution computed tomography (HRCT) scan is the “gold standard” for confirming the presence of bronchiectasis and the extent of disease (14). Figure 1 shows the typical findings seen on HRCT imaging. In order to assess the microbiologic component of the disease a lower respiratory tract sample needs to be sent for cultures and the microbiology needs to be monitored at intervals. Sputum or bronchoalveolar lavage specimens should be cultured for routine bacteria, mycobacteria and fungi.
Evaluation for underlying causes of bronchiectasis
British and European guidelines recommend a routine “bundle” of tests for a patient newly diagnosed with bronchiectasis (14,15). These include a differential blood count, serum immunoglobulins (total IgG, IgA and IgM) and testing for allergic bronchopulmonary aspergillosis (ABPA). Others suggest also checking for alpha one anti-trypsin deficiency, cystic fibrosis, primary ciliary dyskinesia (PCD), autoimmune diseases and chronic aspiration. Bronchoscopy may be indicated, particularly if there is focal bronchiectasis and hence the need to rule out an obstructing airway lesion. There is consensus that a standardized protocol is useful in the initial evaluation but needs to be tailored to the individual patient and informed by taking a detailed history from the patient (7,14). Important elements of the history include the duration of symptoms, the presence or absence of childhood respiratory symptoms, the family and exposure history of the patient and whether the patient has any associated conditions including chronic sinus disease, gastrointestinal or swallowing problems, infertility, auto-immune diseases.
Approach to management of the patient with bronchiectasis
The treatment plan for the bronchiectasis patient should include the following elements as summarized in Table 3: (I) correction of any associated underlying disorder, if possible; (II) attention to general clinical care including education on nutrition, maintaining a healthy lifestyle, receiving appropriate vaccinations (against influenza and pneumococcal infections); (III) airway clearance therapies; (IV) anti-inflammatory therapies, if appropriate; (V) maintenance antibiotics if required; (VI) treatment of exacerbations. A step wise approach to care, tailored to the patient’s clinical condition, severity of disease and infecting micro-organisms, is optimal.
Full table
Correction of underlying disorders
Though rare, patients with bronchiectasis are occasionally found to have previously unsuspected disorders such as primary immunodeficiency or alpha one antitrypsin (A1AT) deficiency. The US Bronchiectasis Research Registry, a database of over 2,000 US patients with bronchiectasis, showed that these conditions were found in a small number (less than 5%) of registrants (19). Treatment of immunodeficiency with immunoglobulin G may reduce the frequency of lower respiratory infections (20); it is less clear how impactful replacement therapy is for A1AT deficiency. Gastro-esophageal reflux, dysphagia and micro-aspiration, commonly present in these patients, can be mitigated by maneuvers including elevation of the head of the bed while sleeping, speech and swallowing therapy and anti-reflux/prokinetic medications. Although it is unclear how much these therapies impact bronchiectasis outcomes, a simple common sense approach may provide benefit.
Though not all patients with bronchiectasis need to be tested for cystic fibrosis, it is important that this disorder be considered in all patients, regardless of age or other demographic findings. Cystic fibrosis (CF) testing should be considered in patients with concomitant sinus disease, gastro-intestinal or pancreatic abnormalities, male infertility and a family history of bronchiectasis. Therapies are now available to help correct cystic fibrosis transmembrane conductance regulator (CFTR) abnormalities and potentially reverse the airway damage in CF; hence it is important to rule out CF as a cause of bronchiectasis.
Airway clearance
Expert consensus opinion is that regular airway clearance therapy should be performed by all patients with clinically significant bronchiectasis (15). Though the scientific evidence is somewhat weak and the optimal modalities are not defined, patients generally report benefit from airway clearance. Mechanical modes of airway clearance include chest physiotherapy with postural drainage, autogenic drainage, active cycle of breathing techniques, huff/forced cough maneuvers. Airway clearance can also be augmented by use of devices such as oscillating positive expiratory pressure (PEP) devices and high frequency chest wall oscillatory “vests”. Routine exercise is also helpful and patients may benefit from enrollment in formal pulmonary rehabilitation programs that include several exercise modalities and patient education. The “Bronchiectasis Toolbox” is a comprehensive website that provides patient instruction on airway clearance (21). Personalizing airway clearance is important in order to optimize adherence (22). Potential complications of airway clearance include oxygen desaturation, chest wall injury and infection due to inadequate device cleaning. Gastrointestinal reflux (GERD) has also been seen in subjects using PEP devices (23) so patients with that co-morbidity need to be cautious so as to not exacerbate their lung disease.
Pharmacologic therapies to improve muco-ciliary transport are also used in patients with bronchiectasis. Though agents like bronchodilators, guaifenesin and n-acetyl cysteine have been historically employed, there is little scientific evidence to support their routine use. Hypertonic saline (7%) has shown benefit in a small study of 32 patients (24); some patient have difficulty tolerating 7% saline but may respond favorably to 3% or 6% concentrations. Two agents used in CF bronchiectasis, rH DNase and inhaled mannitol, have failed to show benefit in patient with bronchiectasis unrelated to CF (25,26). Patients need proper training in the use and maintenance of devices, including nebulizers and compressors. A recommended sequencing of therapies, taken from the CF literature, is to use bronchodilator (if required for co-existent bronchospasm) then hypertonic saline, then airway clearance, followed by inhaled antibiotic (if needed for chronic infection) and then the mucolytic therapy (27).
Anti-inflammatory therapies
Inhaled corticosteroids (ICS) are commonly prescribed in patients with bronchiectasis; a recent study showed that 17% of patients with bronchiectasis in a large US survey group were also diagnosed with asthma and 19% with COPD (28). However, 55% of the overall survey group were chronically prescribed ICS therapy even though the evidence is scant for routine use in bronchiectasis. ICS therapy may be potentially harmful; ICS therapy may increase the rate of pneumonia and a study recently published from Ontario, Canada showed that ICS use is associated with an increased risk of nontuberculous mycobacterial (NTM) infection (29). Oral corticosteroids have no routine role in the treatment of chronic bronchiectasis but occasionally are beneficial when the patient is having an exacerbation that includes bronchospasm.
Statin therapy has been shown to have anti-inflammatory effects in patients with bronchiectasis (30); a recent pilot study of 27 patients with bronchiectasis who were chronically infected with pseudomonas aeruginosa reported an improvement in quality of life but no change in cough severity compared to placebo (31). More study is needed to assess the potential role of statins in patients with bronchiectasis.
Maintenance antibiotics
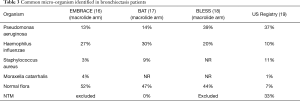
Chronic infection is a hallmark of bronchiectasis; the US Bronchiectasis Research Registry recently reported on 1826 patient with bronchiectasis and found that pseudomonas aeruginosa was the most commonly isolated organism found in 33% of registrants. Staphylococcus aureus and Haemophilus influenza were next in frequency and up to 50% of the subjects were also found to have NTM infections (19). Table 3 summarizes microbiologic data from around the world. When the patient has frequent exacerbations (2 or 3 or more per year), targeted chronic antibiotic strategies may be helpful in reducing exacerbations and possibly improving quality of life.
Macrolide therapy is supported by three clinical trials done in adult patients with bronchiectasis (16-18). In addition to having anti-microbial effects, macrolides also have immunomodulatory properties that appear to benefit patients with bronchiectasis (32). The three published trials used azithromycin 500 mg three times per week (16), azithromycin 250 mg daily (17) and erythromycin ethylsuccinate 400 mg twice daily (18) and all demonstrated benefit versus placebo with regards to reducing the frequency of exacerbations. Some concerns about chronic macrolide therapy include the potential for disrupting the microbiologic ecology of the lung microbiota (33) as well as the development of resistant organisms and adverse effects including hearing loss and cardiac arrhythmias. Additionally, macrolide monotherapy is contraindicated when the patient is also infected with nontuberculous mycobacteria as there is the potential for developing NTM macrolide resistant organisms.
Inhaled antibiotics are attractive as maintenance therapy, particularly when the patient is chronically infected with pseudomonas aeruginosa. However, studies to date have not shown consistent benefit in bronchiectasis. A one year trial of inhaled gentamicin 80 mg twice per day did shown reduction in sputum density and a 31% rate of pseudomonas eradication as well as fewer exacerbations; however the study, though placebo controlled, was not blinded (34). Two studies using inhaled tobramycin 300 mg twice per day showed reduction in sputum density but were not powered to assess clinical benefit and significant side effects including cough and wheeze were seen in those subjects (35,36). Phase II studies on novel inhaled formulations of ciprofloxacin (dry powder and nebulized liposomal) revealed promising results (37,38) but the recent phase III trials did not confirm clinical benefit (39,40) and the US Food and Drug Administration recently rejected the new drug applications for these formulations. Two identical studies that evaluated the effect of inhaled aztreonam for inhalation solution also had negative results (41). Inhaled colistin, which is in common use in Europe and the United Kingdom, also failed to achieve its primary endpoint in a study of 144 patients chronically infected with pseudomonas aeruginosa although there was a signal that it benefitted patients who were most adherent to the treatments (42). Overall, there are many unanswered questions about the efficacy and safety of inhaled antibiotics as chronic maintenance treatments in bronchiectasis. The recent ERS guidelines conditionally recommended, with low quality of evidence, that inhaled antibiotics be considered in patients with chronic pseudomonas infection and a high exacerbation frequency (15). An anecdotal series from a US center also demonstrated the effectiveness of inhaled antibiotics in reducing exacerbations (43).
In certain parts of the world, particularly the United States, NTM infection is a significant pathogen in bronchiectasis patients. The US Bronchiectasis Research Registry reported that 63% of patients had positive NTM cultures or a history of NTM at the time of enrollment in the registry though this number may be an overrepresentation because of selection bias at the enrolling centers (19). However, epidemiologic data has confirmed an increasing prevalence in the US (44). Not all patients with NTM infection require antibiotic treatment but if the organism is persistent and causing lung damage and symptoms then treatment is warranted. Microbiologic results are needed to guide therapy; the most common NTM pathogens are mycobacterium avium complex and mycobacterium abscessus. Guidelines and consensus statements are available to help clinicians decide on which patients to treat and what antimicrobials should be used (45,46).
Treatment of exacerbations in bronchiectasis patients should be guided by culture results from lower respiratory secretions. Mild to moderate exacerbations can usually be treated with an oral antibiotic but if the patient is infected with a drug resistant pseudomonas aeruginosa or other similar pathogen then intravenous antibiotic may be required. Recent expert opinion suggests that 14 days of therapy may be sufficient to treat an exacerbation but this ultimately needs to be assessed by the clinician for the individual patient (15). At present, there are no well-studied biomarkers that can be used to determine when the exacerbation has resolved.
Surgery for bronchiectasis
Surgical resection is a viable option for some patients with bronchiectasis, either to remove the most involved part of the lung or a focal area of disease. Case series have demonstrated the safety of this approach in experienced centers (47,48) and the surgical approach is discussed elsewhere in this volume.
Summary
Bronchiectasis is a resurgent disease in the US and worldwide and patients are being diagnosed with increasing frequency. The medical management of bronchiectasis includes a careful confirmation of the diagnosis based on clinical and computed tomography findings. Patients should have a systematic evaluation of potential causes of bronchiectasis, particularly to assess whether they have a treatable underlying condition which might alter the course of their lung disease. Treatment should be approached in a step wise fashion based on severity of disease and microbiologic findings. A multi-modality approach to treatment is important, adding therapies as dictated by the clinical course. Education of the patient regarding the disease is vitally important. A multidisciplinary care team, including pulmonary physicians, infectious disease and immunology specialists, respiratory care practitioners and pharmacologic experts, is of benefit to patients with this disease. Research trials are ongoing to better elucidate the causes, natural history and best treatments for bronchiectasis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has received honoraria for participating in scientific advisory panels for Bayer Inc., Grifols, Insmed and Electromed. The author has been principal investigator at Georgetown University on studies sponsored by Bayer, Aradigm, Insmed, Parion.
References
- Cole PJ. Inflammation: a two-edged sword—the model of bronchiectasis. Eur J Respir Dis Suppl 1986;147:6-15. [PubMed]
- Laennec RT. On mediate auscultation, or a treatise on the diagnosis of diseases of the lungs and heart. Paris, 1819.
- Barker AF, Bardana EJ. Bronchiectasis: update of an orphan disease. Am Rev Respir Dis 1988;137:969-78. [Crossref] [PubMed]
- Weycker D, Edelsberg J, Oster G, et al. Prevalence and economic burden of bronchiectasis. Clin Pulm Med 2005;12:205-9. [Crossref]
- Seitz AE, Olivier KN, Adjemian J, et al. Trends in bronchiectasis among Medicare beneficiaries in the United States, 2000-2007. Chest 2012;142:432-9. [Crossref] [PubMed]
- Lin JL, Xu JF, Qu JM. Bronchiectasis in China. Ann Am Thorac Soc 2016;13:609-16. [Crossref] [PubMed]
- McShane PJ, Naureckas ET, Tino G, et al. Non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med 2013;188:647-56. [Crossref] [PubMed]
- Field SK, Fisher D, Cowie RL. Mycobacterium avium complex pulmonary disease in patients without HIV infection. Chest 2004;126:566-81. [Crossref] [PubMed]
- Hill AT, Haworth CS, Aliberti S, et al. Pulmonary exacerbation in adults with bronchiectasis: a consensus definition for clinical research. Eur Respir J 2017;49. [Crossref] [PubMed]
- Chalmers JD, Aliberti S, Filonenko A, et al. Characterization of the “frequent exacerbator phenotype” in bronchiectasis. Am J Respir Crit Care Med 2018;197:1410-20. [Crossref] [PubMed]
- Chalmers JD, Goeminne P, Aliberti S, et al. The bronchiectasis severity index. An international derivation and validation study. Am J Respir Crit Care Med 2014;189:576-85. [Crossref] [PubMed]
- Martínez-García MÁ, deGracia J, Vendrell Relat M, et al. Multidimensional approach to non-cystic fibrosis bronchiectasis: the FACED score. Eur Respir J 2014;43:1357-67. [Crossref] [PubMed]
- McDonnell MJ, Aliberti S, Goeminne PC, et al. Comorbidities and the risk of mortality in patients with bronchiectasis: an international multicentre cohort study. Lancet Respir Med 2016;4:969-79. [Crossref] [PubMed]
- Pasteur MC, Bilton D, Hill AT, et al. British Thoracic Society guideline for non-CF bronchiectasis. Thorax 2010;65 Suppl 1:i1-58. [Crossref] [PubMed]
- Polverino E, Goeminne PC, McDonnell MJ, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J 2017;50. [Crossref] [PubMed]
- Wong C, Jayaram L, Karalus N, et al. Azithromycin for prevention of exacerbations in non-cystic fibrosis bronchiectatis (EMBRACE): a randomised, double blind, placebo controlled trial. Lancet 2012;380:660-7. [Crossref] [PubMed]
- Altenburg J, de Graaf CS, Stienstra Y, et al. Effect of azithromycin maintenance treatment on infectious exacerbations among patients with non-cystic fibrosis bronchiectasis: the BAT randomized controlled trial. JAMA 2013;309:1251-9. [Crossref] [PubMed]
- Serisier DJ, Martin ML, McGuckin MA, et al. Effect of long-term, low dose erythromycin on pulmonary exacerbations among patients with non-cystic fibrosis bronchiectasis: the BLESS randomized controlled trial. JAMA 2013;309:1260-7. [Crossref] [PubMed]
- Aksamit TR, O’Donnell AE, Barker A, et al. Adult patients with bronchiectasis. A first look at the US Bronchiectasis Research Registry. Chest 2017;151:982-92. [Crossref] [PubMed]
- Quinti I, Soresina A, Guerra A, et al. Effectiveness of immunoglobulin replacement therapy on clinical outcome in patients with primary antibody deficiencies: results from a multicenter prospective cohort trial. J Clin Immunol 2011;31:315-22. [Crossref] [PubMed]
- Nicolson CH, Holland AE, Lee AL. The Bronchiectasis Toolbox-A Comprehensive Website for the Management of People with Bronchiectasis. Med Sci (Basel) 2017.5. [PubMed]
- McIlwaine M, Bradley J, Elborn JS, et al. Personalising airway clearance in chronic lung disease. Eur Respir Rev 2017;26. [Crossref] [PubMed]
- Lee AL, Denehy L, Wilson JW, et al. Upright positive pressure therapy and exercise: effects on gastroesophageal reflux in COPD and bronchiectasis. Respir Care 2012;57:1460-7. [Crossref] [PubMed]
- Kellett F, Robert NM. Nebulized 7% hypertonic saline improves lung function and quality of life in bronchiectasis. Respir Med 2011;105:1831-5. [Crossref] [PubMed]
- O’Donnell AE, Barker AF, Ilowite JS, et al. Treatment of idiopathic bronchiectasis with aerosolized recombinant DNase I rh DNase Study Group. Chest 1998;113:1329-34. [Crossref] [PubMed]
- Bilton D, Tino G, Chambers DC, et al. Inhaled mannitol for non-cystic fibrosis bronchiectasis: a randomized controlled trial. Thorax 2014;69:1073-9. [Crossref] [PubMed]
- Agent P, Parrott H. Inhaled therapy in cystic fibrosis: agents, devices and regimens. Breathe (Sheff) 2015;11:110-8. [Crossref] [PubMed]
- Henkle E, Aksamit TR, Barker AF, et al. Pharmacotherapy for non-cystic fibrosis bronchiectasis. Results from an NTM Info and Research Patient survey and the Bronchiectasis and NTM Research Registry. Chest 2017;152:1120-7. [Crossref] [PubMed]
- Brode SK, Campitelli MA, Kwong JC, et al. The risk of mycobacterial infections associated with inhaled corticosteroid use. Eur Respir J 2017;50. [Crossref] [PubMed]
- Mandal P, Chalmers JD, Graham C, et al. Atorvastatin as a stable treatment in bronchiectasis: a randomised controlled trial. Lancet Respir Med 2014;2:455-63. [Crossref] [PubMed]
- Bedi P, Chalmers JD, Graham C, et al. A randomized controlled trial of atorvastatin in patients with bronchiectasis infected with pseudomonas aeruginosa. A proof of concept study. Chest 2017;152:368-78. [Crossref] [PubMed]
- Kanoh S, Rubin BK. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin Microbiol Rev 2010;23:590-615. [Crossref] [PubMed]
- Rogers GB, Bruce KD, Martin ML, et al. The effect of long-term macrolide treatment on respiratory microbiota composition in non-cystic fibrosis bronchiectasis: an analysis from the randomised, double blind, placebo controlled BLESS trial. Lancet Respir Med 2014;2:988-96. [Crossref] [PubMed]
- Murray MP, Govan JR, Doherty CJ, et al. A randomized controlled trial of nebulized gentamicin in non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med 2011;183:491-9. [Crossref] [PubMed]
- Barker AF, Cough L, Fiel SB, et al. Tobramycin solution for inhalation reduces sputum Pseudomonas aeruginosa density in bronchiectasis. Am J Respir Crit Care Med 2000;162:481-5. [Crossref] [PubMed]
- Scheinberg P, Shore E. A pilot study of the safety and efficacy of tobramycin solution for inhalation in patients with severe bronchiectasis. Chest 2005;127:1420-6. [PubMed]
- Wilson R, Welte T, Polverino E, et al. Ciprofloxacin dry powder for inhalation in noncystic fibrosis bronchiectasis: a phase II randomised study. Eur Respir J 2013;41:1107-15. [Crossref] [PubMed]
- Serisier DJ, Bilton D, deSoyza A, et al. Inhaled, dual release liposomal ciprofloxacin in noncystic fibrosis bronchiectasis (ORBIT-2): a randomised, double blind, placebo controlled trial. Thorax 2013;68:812-17. [Crossref] [PubMed]
- Aksamit T, De Soyza A, Bandel TJ, et al. RESPIRE 2: a phase III placebo-controlled randomised trial of ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis. Eur Respir J 2018;51. [Crossref] [PubMed]
- Haworth C, Wanner A, Froehlich J, et al. Inhaled liposomal ciprofloxacin in patients with bronchiectasis and chronic pseudomonas aeruginosa infections: results from two parallel phase III trials (ORBIT-3 and ORBIT-4. Am J Respir Crit Care Med 2017;195:A7604.
- Barker AF, O’Donnell AE, Flume P, et al. Aztreonam for inhalation solution in patients with non-cystic fibrosis bronchiectasis (AIR-BX1 and AIR-BX2): two randomised double-blind, placebo-controlled phase 3 trials. Lancet Respir Med 2014;2:738-49. [Crossref] [PubMed]
- Haworth CS, Foweraker JE, Wilkinson P, et al. Inhaled colistin in patients with bronchiectasis and chronic pseudomonas infection. Am J Respir Crit Care Med 2014;189:975-82. [Crossref] [PubMed]
- Nadig TR, Flume PA. Aerosolized antibiotics for patients with bronchiectasis. Am J Respir Crit Care Med 2016;193:808-10. [Crossref] [PubMed]
- Adjemian J, Oliver KN, Seitz AE, et al. Prevalence of nontuberculous mycobacterial disease in US Medicare beneficiaries. Am J Respir Crit Care Med 2012;185:881-6. [Crossref] [PubMed]
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007;175:367-416. [Crossref] [PubMed]
- Novosad S, Henkle E, Winthrop KL. The challenge of pulmonary nontuberculous mycobacterial infection. Curr Pulmonol Rep 2015;4:152-61. [Crossref] [PubMed]
- Dai J, Zhu X, Bian D, et al. Surgery for predominant lesion in nonlocalized bronchiectasis. J Thorac Cardiovasc Surg 2017;153:979-85. [Crossref] [PubMed]
- Yu JA, Weyant MJ, Mitchell JD. Surgical treatment of atypical mycobacterial infections. Thorac Surg Clin 2012;22:277-85. [Crossref] [PubMed]



