Afferent neural pathways mediating cough in animals and humans
Overview of the primary afferent innervation of the airways and lungs
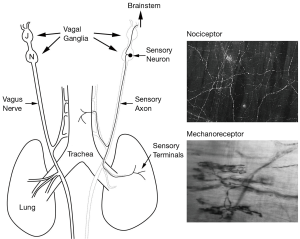
The afferent innervation to the airways and lungs responsible for mediating cough is derived exclusively from either the nodose (inferior) or jugular (superior) vagal ganglia (Figure 1). Although it can be debated how pulmonary vagal afferents are best classified (i.e., whether it should be based on their physiological, anatomical and/ or molecular characteristics), it is generally agreed that subsets of vagal sensory nerves, differentiated by their responsiveness to either mechanical or chemical stimuli, are distributed throughout the pulmonary system (1). Pulmonary mechanosensors are exclusively derived from the nodose ganglia and are classically identified as either rapidly (RAR) or slowly (SAR) adapting airway mechanoreceptors based on their exquisite sensitivity to stretch (inflation and deflation) of the lungs and their adaptation properties during a sustained lung inflation (1). RARs and SARs are perhaps best known for their role in the Herring-Breuer reflex regulation of respiration, but additionally these airway mechanoreceptors play important roles in the regulation of bronchomotor tone and other airway reflexes (2-4). By contrast, pulmonary chemosensors are derived from both the nodose and jugular ganglia and are characterised by their insensitivity to lung stretch but sensitivity to a wide variety of chemical agents which stereotypically includes capsaicin from hot chilli peppers (5,6). Chemosensors are largely quiescent in normal airways, but are recruited during irritation or inflammation of the airways. Because chemosensors are typically only activated by noxious stimuli in the pulmonary system they are often referred to as nociceptors, and both C- and Adelta-fiber subtypes have been identified in many species. Within this broad classification of pulmonary afferents, additional subtypes of mechano- and chemo-sensitive fibers can be identified, and this has been reviewed elsewhere in detail (1,7).
A dual afferent model subserving cough
Historically there has been some debate over which subtypes of pulmonary afferent nerves are responsible for the initiation and regulation of coughing. A substantial body of literature exists describing cough evoked by chemosensors, reflecting the fact that chemical stimuli (such as capsaicin, bradykinin and citric acid inhalation) are effective at inducing cough (1). Indeed, C-fibre nerve terminals densely innervate the epithelium, often sandwiched between epithelial cells and in close apposition to the airway lumen (7,8). Thus they are ideally anatomically positioned to detect noxious substances entering the airways from the external environment. However, nociceptor stimulants do not evoke cough in animals or humans under general anaesthesia, and in some circumstances C-fibre stimulation is inhibitory to cough (4,9). For these reasons, the role of C-fibres in cough may seem questionable. However, these conflicting observations may in fact suggest that cortical processing of chemosensor stimuli is necessary for conscious perception of airway irritation, and that this facilitates behavioural rather than reflex coughing (discussed below).
Mechanical stimuli (such as touching the epithelial surface of large airways) are also effective at evoking cough, and because chemosensors are not readily activated by mechanical stimulation it has often been assumed that RARs (sometimes termed ‘irritant receptors’ in the cough literature) mediate mechanical cough. However, arguably much of the literature describing RAR-evoked cough needs to be interpreted with care. This in large part reflects the fact that stimuli that truly activate RARs (such as lung collapse and bronchospastic agents) are simply ineffective at evoking cough (4,10). This discrepancy remained a conundrum in the field until recently when a novel subtype of rapidly adapting mechanosensor (termed the “cough receptor”) was discovered in the large airways of guinea pigs (4). Cough receptors are characterized as extrapulmonary, low threshold Adelta-fibre mechanosensors with rapidly adapting properties, but can be distinguished from classical RARs by (I) their unresponsiveness to tissue stretch (instead responding to punctate mechanical stimuli); (II) conduction velocity (classic RARs are Abeta fibers); and (III) unresponsiveness to purinergic agonists (cough receptors don’t express purinergic P2X receptors whereas classic RARs do). Originating from the nodose ganglia of the vagus nerve (11,12), cough receptors do not contain neuropeptides, but rather express markers of glutamatergic neurons as well as uniquely expressing alpha3-containing isozymes of the sodium pump (Na+/K+ ATPase) (11,13). Cough receptor terminals sit beneath the airway epithelium, but above the airway smooth muscle of the larynx, trachea and primary bronchi (11), in a position that is suited to detecting mechanical stimuli applied to the epithelial surface (such as inhaled particulate matter). They are also readily distinguished anatomically from chemosensors in the airway wall because of their characteristic nerve terminal arrangements (Figure 1).
The existence of two distinct afferent pathways mediating cough undoubtedly reflects the range of potential stimuli that can cause irritation or damage to the airways. This of course includes inhaled (environmental) chemicals, intrinsically produced chemicals (such as inflammatory mediators), aspirated or inhaled particulate matter and/or gastric contents, and airway mucous or other secretions. These two subsets of primary afferents express numerous channels and receptors that are responsible for detecting specific stimuli. Airway chemosensors express TRPV1 and TRPA1 which combined equip nociceptors with capacity to detect a host of stimuli including but not limited to capsaicin, acid, alcohol, acrolein, allyl isothiocynate (AITC), as well as making nociceptors temperature responsive (14). Chemosensors also express a number of G-protein coupled receptors, most notably receptors for bradykinin, prostaglandin E2, adenosine and serotonin (14). By contrast, less is known about the molecular machinery of cough receptors. The mechanically gated ion channel has yet to be described, but unpublished data from our laboratory indicates that mechanically evoked membrane currents expressed by cough receptors are inhibited by amiloride and the non-selective TRP channel blocker ruthenium red and not by the stretch activated channel blocker gadolinium. Cough receptors are also activated by rapid reductions in pH (15), an effect that is likely mediated by one or more members of the acid sensitive ion channels (ASICs) as cough receptors do not express TRPV1 under normal conditions (16). Data from our laboratory has additionally shown that cough receptors express the furosemide sensitive sodium potassium chloride co-transporter (NKCC1) that is involved in responses mediated by hypotonic solutions (17). The list of known receptors and channels that mediate responses in chemosensors and cough receptors is almost certainly incomplete and more work is needed in this area, which may identify novel therapeutic targets for cough suppression.
Sensory inputs to the central nervous system: reflex versus behavioural cough
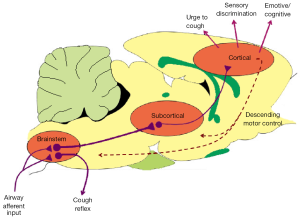
Cough is typically thought of as a reflex, requiring primary afferent input to the brainstem leading to reflexive changes in respiration. However, this simplistic view is not sufficient to explain all types of cough. Indeed, cough can either occur reflexively (requiring minimal conscious involvement) or behaviorally (requiring higher brain cortical processing) (4,18,19). Furthermore, cough is accompanied by complex sensory and cognitive processes in the higher brain that give rise to feelings such as the urge-to-cough, adding an additional dimension to the nature of problematic cough in disease (13,20). The neurobiology of reflexive and behavioural cough processes is only beginning to be described (Figure 2).
Reflex cough is largely dependent on afferent inputs processed at the brainstem level and can be evoked from anesthetised or decerebrated animals (1,4,18) suggesting higher neural processing is not essential. Brainstem processing of airways sensory information is thus the minimum level of processing required for basic defense and airway clearance. However, activation of peripheral sensory nerves in the airways is not sufficient of itself to evoke coughing. Indeed, cough occurs as a result of a reconfiguration of the brainstem respiratory pattern generator in such a way that normal respiratory rhythm is replaced by a cough motor pattern (18). Accordingly the nature of the sensory inputs to the central nervous system is important for evoking reflex cough. Consistent with this, recent studies have described the encoding of primary afferent information for airway mechanosensitive pathways that is necessary to induce coughing, which typically requires high firing rates along cough receptor axons (21,22). Indeed, the unique expression of the alpha3-containing isozymes of (Na+/K+ ATPase by cough receptors is essential in sustaining high frequency firing (21).
How chemosensors encode for cough is not clear, but the available evidence might suggest that chemosensors encode for cough by promoting behavioural rather than reflexive coughing. Thus, chemical irritants (such as capsaicin) readily induce the urge-to-cough when inhaled and capsaicin-evoked cough and the urge-to-cough is highly amenable to voluntary or suggestive controls, including conscious cough suppression and placebo inhibition (13,19,23-27). Furthermore, whilst mechanosenor-evoked reflexive cough is inducible in decerebrated animals or under general anaesthesia, chemosensor-evoked coughing is not. Thus it may be that incoming chemosensory inputs, particularly at modest stimulus intensities, are relayed to higher brain regions to induce the perception of airway irritation and the resultant cough response includes descending motor drives from the cortex to respiratory centres in the lower brain (19,28) (Figure 2).
Neuroanatomy of cough pathways in the brain: animal studies
Chemosensitive and mechanosensitive airway afferent nerves provide inputs to the brainstem which are widely considered to terminate primarily at the level of the nucleus of the solitary tract (nTS) (28-32). However, recent studies suggest, that a substantial portion of airway sensory inputs also terminate in a region of the trigeminal nucleus known as the paratrigeminal nucleus (Pa5) (31,32). Animal studies have revealed the caudal nTS to be a complex integration region for a variety of visceral sensory processes. Indeed, the nTS provides an extensive array of ascending and descending projections to numerous regions throughout the brain, brainstem and spinal cord, including well described inputs to cardiorespiratory nuclei in the ventrolateral medulla. Although the identity of the nTS neurons that are in receipt of cough-specific airway sensory inputs have not been described, a variety of studies have reported the nTS subnuclei that are critical for coughing. Thus in several species, neuroanatomical tracing, electrical stimulation of the brainstem, microinjection studies and/or noxious airway challenges resulting in the expression of the neuronal activation marker (c-FOS) have been used to describe airway afferent integration sites within the dorsolateral, medial and commissural nTS (31,33-36) With respect to the basic cough reflex, neurons arising from the nTS project to the ventral respiratory group, the functional organisation of which has been described in detail (18). Nothing is known about potential projections from the Pa5 to the ventral respiratory group with respect to coughing.
Unlike reflexive cough, behavioural cough requires airway sensory inputs to reach higher brain circuits responsible for sensory perception and planning/initiating motor control over respiratory muscles (Figure 2). To achieve this, second order neurons from brainstem integration nuclei must relay information to higher order brain nuclei, as airway afferents do not directly project to supramedullary sites (i.e., regions above the brainstem).The organisation of this higher brain sensory circuitry was, for a long time, unknown. However recent studies have begun to describe the pathways via which airway afferents can modulate cortical responses. Using herpes virus, which infects sensory neurons and migrates specifically along ascending sensory pathways, we have described in detail the central organisation of airway sensory projections in the higher brain. Thus, airway sensory neurons project via the nTS and Pa5 via at least two ascending pathways to the cortex (31,32). The first is a trigeminothalamocortical pathway that includes terminations in the ventrobasal thalamus and projects to the somatosensory cortices. The second is a thalamolimbic pathway that has terminations in the mediodorsal thalamus, submedius thalamus, lateral hypothalamus and projects to limbic and paralimbic cortices (anterior insula, cingulate and orbital cortices).
These distinct ascending afferent pathways likely contribute to the perception of airway irritation and the cognitive, emotional and behavioural consequences that arise as a result (e.g., drive to respond). In fact, the behavioural responses of animals to aversive respiratory stimuli are indicative of conscious awareness. Cats have been shown to behaviourally modulate breathing (37-40) and rats demonstrated a conditional aversion to hypoxia and hypercapnia (41,42), and breathing against resistive loads or noxious stimulation of the airways results in activity (altered gene expression, for example) in many of the brain regions revealed by herpes viral neuronal tracing (43,44). Furthermore, electrical stimulation of brain regions such as the orbital cortex and amygdala in cats has modulating effects on evoked coughing (45,46).
Neuroanatomy of cough pathways in the brain: human studies
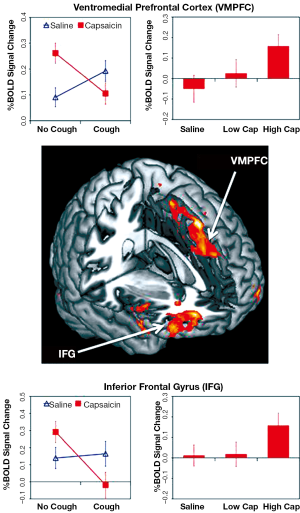
A difficulty with animal studies, such as those described above, is relating the neuroanatomical and neurophysiological outcomes with behavioural responses that are subjective in nature (such as the urge-to-cough). For this reason, we have turned to functional brain imaging approaches in humans to gain a better insight into the organisation of neuronal elements that govern complex behaviours associated with airways irritation (13,19,21,23,47). Inhalation of capsaicin in healthy humans evokes activations in a distributed brain network, which includes somatosensory, motor, prefrontal and limbic cortices. By modifying the concentration of capsaicin (stimulus intensity) such that the magnitude of the participants’ subjective experience (urge-to-cough) was varied, we have identified distinct activation patterns within the distributed brain network that may encode for stimulus intensity (insula cortex), perceptual magnitude (somatosensory cortex), and attention/stimulus localisation (posterior parietal cortex) (27). Notably, many of the regions activated by capsaicin share homology with the brain nuclei identified in animal studies (31). We have also described regional responses that are associated with cough and cough suppression, the former involving activity in cortical motor and premotor regions, while the latter includes responses in the ventromedial prefrontal cortex and inferior frontal gyrus, a network that has been described previously as playing a role in inhibiting motor responses (19,48) (Figure 3). Interestingly, both the urge-to-cough and the associated capsaicin-evoked responses in the brain are modifiable by placebo administration (24,25). Thus the belief that an antitussive therapeutic will reduce the magnitude of the urge-to-cough, results in a reduced urge-to-cough relative to the stimulus magnitude, less associated brain activity in regions that are believed to encode sensory discrimination, and recruitment of the dorsolateral prefrontal cortex, a region that has been implicated previously in placebo suppression. Additional studies are needed to understand how the dorsolateral prefrontal cortex modifies incoming sensory information from the airways.
Conclusions
Cough is a complex neurobiological process that is dependent on sensory processes that begin in the airways and include multiple pathways acting at all levels of the central nervous system. Whilst we now know a substantial amount about the nature of the primary sensory inputs to the central nervous system and the organisation of cough networks in the brainstem and brain, many gaps in our knowledge remain. For example, we do not know whether differences exist in the higher brain organisation of inputs from different sensory neuron subtypes. More work also needs to be done to map the connections of brain regions receiving inputs from the airways, and to identify responses in subcortical and brainstem regions in humans (the imaging performed to date has been optimised for cortical responses). Furthermore, understanding how these networks behave in patients with dysfunctional cough will be important. By addressing these questions we hope to identify sites along the sensory cough pathways that provide opportunities for therapeutic intervention and cough suppression.
Acknowledgements
Funding: Funded by National Health and Medical Research Council of Australia (grants 1042528 and 1025589).
Disclosure: The authors declare no conflict of interest.
References
- Mazzone SB. An overview of the sensory receptors regulating cough. Cough 2005;1:2. [PubMed]
- Mazzone SB, Canning BJ. Evidence for differential reflex regulation of cholinergic and noncholinergic parasympathetic nerves innervating the airways. Am J Respir Crit Care Med 2002;165:1076-83. [PubMed]
- Mazzone SB, Canning BJ. An in vivo guinea pig preparation for studying the autonomic regulation of airway smooth muscle tone. Auton Neurosci 2002;99:91-101. [PubMed]
- Canning BJ, Mazzone SB, Meeker SN, et al. Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetized guinea-pigs. J Physiol 2004;557:543-58. [PubMed]
- Undem BJ, Chuaychoo B, Lee MG, et al. Subtypes of vagal afferent C-fibres in guinea-pig lungs. J Physiol 2004;556:905-17. [PubMed]
- Ho CY, Gu Q, Lin YS, et al. Sensitivity of vagal afferent endings to chemical irritants in the rat lung. Respir Physiol 2001;127:113-24. [PubMed]
- Canning BJ, Mori N, Mazzone SB. Vagal afferent nerves regulating the cough reflex. Respir Physiol Neurobiol 2006;152:223-42. [PubMed]
- Baluk P, Gabella G. Afferent nerve endings in the tracheal muscle of guinea-pigs and rats. Anat Embryol 1991;183:81-7. [PubMed]
- Tatar M, Webber SE, Widdicombe JG. Lung C-fibre receptor activation and defensive reflexes in anaesthetized cats. J Physiol 1988;402:411-20. [PubMed]
- Chou YL, Scarupa MD, Mori N, et al. Differential effects of airway afferent nerve subtypes on cough and respiration in anesthetized guinea pigs. Am J Physiol Regul Integr Comp Physiol 2008;295:R1572-84. [PubMed]
- Mazzone SB, Reynolds SM, Mori N, et al. Selective expression of a sodium pump isozyme by cough receptors and evidence for its essential role in regulating cough. J Neurosci 2009;29:13662-71. [PubMed]
- Ricco MM, Kummer W, Biglari B, et al. Interganglionic segregation of distinct vagal afferent fibre phenotypes in guinea-pig airways. J Physiol 1996;496:521-30. [PubMed]
- Mazzone SB, McGovern AE. Immunohistochemical characterization of nodose cough receptor neurons projecting to the trachea of guinea pigs. Cough 2008;4:9. [PubMed]
- Mazzone SB, Undem BJ. Cough sensors. V. Pharmacological modulation of cough sensors. Handb Exp Pharmacol 2009;187:99-127. [PubMed]
- Kollarik M, Undem BJ. Mechanisms of acid-induced activation of airway afferent nerve fibres in guinea-pig. J Physiol 2002;543:591-600. [PubMed]
- Lieu TM, Myers AC, Meeker S, et al. TRPV1 induction in airway vagal low-threshold mechanosensory neurons by allergen challenge and neurotrophic factors. Am J Physiol Lung Cell Mol Physiol 2012;302:L941-8. [PubMed]
- Mazzone SB, McGovern AE. Na+-K+-2Cl- cotransporters and Cl- channels regulate citric acid cough in guinea pigs. J Appl Physiol 2006;101:635-43. [PubMed]
- Rybak IA, O’Connor R, Ross A, et al. Reconfiguration of the pontomedullary respiratory network: a computational modeling study with coordinated in vivo experiments. J Neurophysiol 2008;100:1770-99. [PubMed]
- Mazzone SB, Cole LJ, Ando A, et al. Investigation of the neural control of cough and cough suppression in humans using functional brain imaging. J Neurosci 2011;31:2948-58. [PubMed]
- Davenport PW. Clinical cough I: the urge-to-cough: a respiratory sensation. Handb Exp Pharmacol 2009;187:263-76. [PubMed]
- Mazzone SB, McGovern AE, Koo K, et al. Mapping supramedullary pathways involved in cough using functional brain imaging: comparison with pain. Pulm Pharmacol Ther 2009;22:90-6. [PubMed]
- Canning BJ, Mori N. Encoding of the cough reflex in anesthetized guinea pigs. Am J Physiol Regul Integr Comp Physiol 2011;300:R369-77. [PubMed]
- Farrell MJ, Cole LJ, Chiapoco D, et al. Neural correlates coding stimulus level and perception of capsaicin-evoked urge-to-cough in humans. Neuroimage 2012;61:1324-35. [PubMed]
- Leech J, Mazzone SB, Farrell MJ. The effect of placebo conditioning on capsaicin-evoked urge to cough. Chest 2012;142:951-7. [PubMed]
- Leech J, Mazzone SB, Farrell MJ. Brain activity associated with placebo suppression of the urge-to-cough in humans. Am J Respir Crit Care Med 2013;188:1069-75. [PubMed]
- Hegland KW, Bolser DC, Davenport PW. Volitional control of reflex cough. J Appl Physiol 2012;113:39-46. [PubMed]
- Hutchings HA, Morris S, Eccles R, et al. Voluntary suppression of cough induced by inhalation of capsaicin in healthy volunteers. Respir Med 1993;87:379-82. [PubMed]
- Simonyan K, Saad ZS, Loucks TM, et al. Functional neuroanatomy of human voluntary cough and sniff production. Neuroimage 2007;37:401-9. [PubMed]
- Jordan D. Central nervous pathways and control of the airways. Respir Physiol 2001;125:67-81. [PubMed]
- Kubin L, Alheid GF, Zuperku EJ, et al. Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol 2006;101:618-27. [PubMed]
- McGovern AE, Davis-Poynter N, Farrell MJ, et al. Transneuronal tracing of airways-related sensory circuitry using herpes simplex virus 1, strain H129. Neuroscience 2012;207:148-66. [PubMed]
- McGovern AE, Davis-Poynter N, Rakoczy J, et al. Anterograde neuronal circuit tracing using a genetically modified herpes simplex virus expressing EGFP. J Neurosci Methods 2012;209:158-67. [PubMed]
- Canning BJ, Mori N. An essential component to brainstem cough gating identified in anesthetized guinea pigs. FASEB J 2010;24:3916-26. [PubMed]
- Ohi Y, Yamazaki H, Takeda R, et al. Functional and morphological organization of the nucleus tractus solitarius in the fictive cough reflex of guinea pigs. Neurosci Res 2005;53:201-9. [PubMed]
- Davenport PW, Vovk A. Cortical and subcortical central neural pathways in respiratory sensations. Respir Physiol Neurobiol 2009;167:72-86. [PubMed]
- Canning BJ. Central regulation of the cough reflex: therapeutic implications. Pulm Pharmacol Ther 2009;22:75-81. [PubMed]
- Orem J, Netick A. Behavioral control of breathing in the cat. Brain Res 1986;366:238-53. [PubMed]
- Orem J. The activity of late inspiratory cells during the behavioral inhibition of inspiration. Brain Res 1988;458:224-30. [PubMed]
- Orem J. Behavioral inspiratory inhibition: inactivated and activated respiratory cells. J Neurophysiol 1989;62:1069-78. [PubMed]
- Davenport PW, Dalziel DJ, Webb B, et al. Inspiratory resistive load detection in conscious dogs. J Appl Physiol 1991;70:1284-9. [PubMed]
- Nsegbe E, Vardon G, Perruchet P, et al. Classic conditioning of the ventilatory responses in rats. J Appl Physiol 1997;83:1174-83. [PubMed]
- Nsegbe E, Villaret E, Renolleau S, et al. Behavioural correlates of conditioned ventilatory responses to hypoxia in rats. Behav Brain Res 1999;106:29-37. [PubMed]
- Pate KM, Davenport PW. Tracheal occlusions evoke respiratory load compensation and neural activation in anesthetized rats. J Appl Physiol 2012;112:435-42. [PubMed]
- Bernhardt V, Hotchkiss MT, Garcia-Reyero N, et al. Tracheal occlusion conditioning in conscious rats modulates gene expression profile of medial thalamus. Front Physiol 2011;2:24. [PubMed]
- Kasé Y, Kito G, Miyata T, et al. Influence of cerebral cortex stimulation upon cough-like spasmodic expiratory response (SER) and cough in the cat. Brain Res 1984;306:293-8. [PubMed]
- Kito G, Kasé Y, Miyata T, et al. Neural mechanism for production of spasmodic expiratory response like cough induced by amygdala stimulation in the cat. I. Pathways from the amygdala to the lower brain stem. Arch Int Pharmacodyn Ther 1977;229:116-28. [PubMed]
- Mazzone SB, McGovern AE, Yang SK, et al. Sensorimotor circuitry involved in the higher brain control of coughing. Cough 2013;9:7. [PubMed]
- Jahfari S, Waldorp L, van den Wildenberg WP, et al. Effective connectivity reveals important roles for both the hyperdirect (fronto-subthalamic) and the indirect (fronto-striatal-pallidal) fronto-basal ganglia pathways during response inhibition. J Neurosci 2011;31:6891-9. [PubMed]


