Simultaneously surgical management of adult complex coarctation of aorta concomitant with intracardiac abnormality
Introduction
Coarctation of aorta (COA) is one of the most common congenital heart diseases, the incidence of which is about 6−7%. In addition, coarctation of the aorta is usually accompanied by other cardiac defects, such as aortic bicuspid valve, ventricular septal defect (VSD) and so on. As patients get older, it is easier for them to be affected by acquired heart diseases (1). In fact, untreated patients are prone to early development of severe hypertension and heart failure. Nevertheless, patients with complex coarctation of the aorta concomitant with intracardiac abnormality still pose a difficult surgical problem. Although several surgical approaches have been described for the management of these patients, including a staged procedure using both median sternotomy and left thoracotomy, a catheter-based intervention of the coarctation combined with sternotomy has proved to be an alternative treatment. Besides, various extra-anatomic aortic graft bypass techniques have been preferred in the treatment of complex coarctation of the aorta; connecting the ascending aorta to the descending thoracic aorta, the supraceliac abdominal aorta, or the infrarenal abdominal aorta (2-4). Yet up until now, there has been no universal consensus on an optimal way to manage complex coarctation of the aorta. We retrospectively reviewed departmental cases of extra-anatomic aortic bypass grafting and simultaneous intracardiac abnormality repair in dealing with complex coarctation of the aorta concomitant with intracardiac abnormalities, aiming to provide recommendations for effective and safe treatment.
Methods
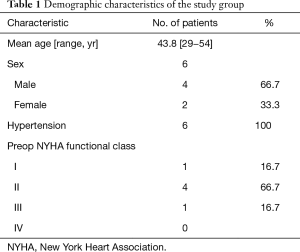
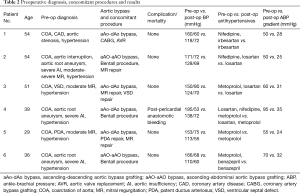
This study was approved by the Nanjing Medical University Institutional Review Board (No. 2016-SR-144). A waiver of the need to obtain consent from patients was approved. From May 2012 to June 2017, we retrospectively reviewed a total of six adult cases concerning complex coarctation of the aorta concomitant with intracardiac abnormality, from the records of the first affiliated hospital with Nanjing Medical University, among whom 4 patients were male and 2 patients were female, the age range being 43.8±10.6 years old (Table 1). All patients suffered from hypertension with preoperative upper and lower limb blood pressure gradients of 63.3±17.2 mmHg. All of them received oral anti-hypertensive drugs. The intracardiac diagnosis of all patients were confirmed by echocardiography. The diagnosis of COA among all patients were confirmed by Chest X-ray as well as Chest CT angiography. The CT angiography showed the coarctation of all patients were located in the aortic isthmus with diameters of 9.5±5 mm. In three patients the condition was accompanied by aortic arch dysplasia and another patient was co-morbid with aortic interruption. The chest X-ray of one patient demonstrated a prominent aortic 3 figure sign and rib erosion (Figure 1). Three patients were analyzed by coronary artery angiography. The associated intracardiac abmonalities diagnosed by echocardiography were 3 aortic root aneurysms, 3 aortic insufficiencies (AI), 1 aortic stenosis, 3 mitral regurgitation (MR), 1 coronary artery disease (CAD), 1 patent ductus arteriosus (PDA) and 1 VSD (Table 2).
Full table
Full table
Surgical procedures applied for the extra-anatomic aortic bypass grafting comprised 4 ascending-descending aortic bypass grafting and 2 ascending-abdominal aortic bypass grafting. Simultaneous intracardiac procedures included 3 Bentall procedures, 1 AVR, 3 mitral valve repairs, 1 CABG, 1 PDA repair and 1 VSD repair (Table 2).
The diagnostic criteria for COA is mainly based on upper-lower limb peak gradient and the chest CT angiography. The peak gradient greater than 20 mmHg and a greater than 50% decrease in diameter at the aortic isthmus should be considered severe coarctation.
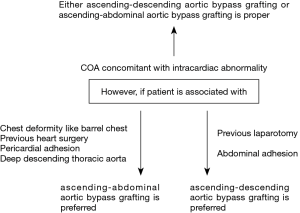
The indications for extra-anatomic aortic bypass grafting in this series include two conditions. Firstly, coarctation with cardiac problems that required repair through median sternotomy or median sternotomy-laparotomy. Secondly, complex coarctation or re-coarctation, for which extra-anatomic bypass grafting was chosen because of the anticipated difficulties with direct anatomic repair.
The contraindication for extra-anatomic aortic bypass grafting is younger patients without concomitant cardiac anomalies, especially children, because they are still growing.
Operative technique
Ascending to descending aortic bypass grafting (aAo-dAo bypass): the procedure was carried out by median sternotomy under cardiopulmonary bypass. After the cardiopulmonary bypass was established by aortic and right atrial or bicaval cannulation, the cardiac apex was retracted cephalad to ascertain the optimal exposure of thoracic descending aorta and a longitudinal pericardial incision was made directly over the descending thoracic aorta. The thoracic aorta behind the pericardium was exposed and partially clamped. Next, an 18−22 mm Dacron graft was employed for an end-to-side anastomosis to thoracic descending aorta using a continuous 4-0 polypropylene suture. The graft was then inserted between the inferior vena cava and the right inferior pulmonary vein. Subsequently, the heart was arrested and the intracardiac abnormality was repaired. After de-airing the right atrium, the Dacron graft was end-to-side anastomosed with the right side of the ascending aorta (Figure 2).
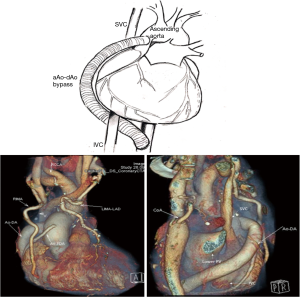
Ascending to abdominal aortic bypass grafting (aAO-aAO bypass): the procedure was done by median sternotomy and laparotomy. A midline incision was made from the suprasternal notch to a point about half-way between the navel and the pubis. The infra-renal abdominal aorta needed to be exposed and partially clamped. An end-to-side 18 mm Dacron graft was employed to establish an infra-renal abdominal aorta anastomosis, using continuous 4-0 polypropylene suture. Care was taken to ensure that the anastomotic site was covered with retroperitoneal peritoneum. The graft was routed behind the transverse colon and stomach, then penetrated through the small omental sac and inserted into the mediastinum or right thorax through the diaphragm. The heart was arrested and the intracardiac abnormality was repaired. After de-airing, the heart was reperfused to beat. Finally, the proximal part of the Dacron graft was end-to-side anastomosed with the ascending aorta (Figure 3).
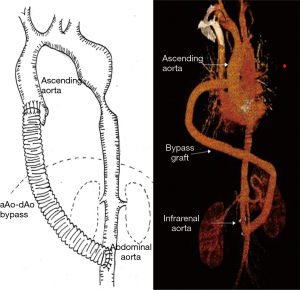
All surgical plans were determined by the pre-operative consensus of surgeons. Generally, either ascending-descending aortic bypass grafting or ascending-abdominal aortic bypass grafting was applied. To determine which procedure should be employed, specific situations should be carefully considered. For example, it is preferred to select ascending-descending aortic bypass grafting for patients with abdominal adhesion. The details behind the choices of surgical procedure are shown in Figure 4.
Statistical analysis
All statistical analyses were done using computer software (SPSS; Chicago, IL, USA). All data was expressed as mean ±S. A value of P<0.05 was considered statistically significant.
Results
No early or late mortality has occurred. All patients were free from cerebral and spinal complications such as stroke and paraplegia. Only one patient receiving ascending-descending aortic bypass grafting needed reexploration for hemostasis because of post-pericardial anastomosis bleeding. Meanwhile the same patient suffered from acute renal failure, however the renal function was completely recovered after a 7-day hemodialysis. All other patients had uneventful recoveries.
For all patients, the intraoperative cardiac arrest time was 175±46.7 minutes; intraoperative cardiopulmonary bypass time was 204.8±66 minutes; post-op extubation time was 32.1±24.8 hours; post-op chest tube drainage time was 7.5±3.2 days.
Post-op blood pressure dramatically decreased
Post-op blood pressure decreased up to 41±10 mmHg (pre-op blood pressure 165.2±16.3 mmHg; VS post-op blood pressure 121.5±10.8 mmHg; P<0.05).
Ankle-brachial pressure gradient significantly improved
All patients had ankle-brachial pressure gradients significantly reduced (pre-operative 63.3±17.2 mmHg versus post-operative 29.1±4.3 mmHg; P<0.05).
The follow-up time ranged from 2 month to 5 years, with a mean follow-up time of 37±22.9 months. All patients survived and their blood pressure decreased to normal range with fewer oral anti-hypertensive drugs compared with pre-surgery. The bypass grafts were patent without any complications. All patients are free from pre-operative symptoms.
Discussion
COA is one of the most common congenital cardiovascular pathologies, with an incidence rate of about 6.5% (5). The occurrence of complex COA accompanied by other intracardiac abnormality is rare, reportedly less than 1% (6). The most common congenital intracardiac pathologies associated with complex COA are bicuspid aortic valve, atrial septal defect, VSD, PDA, and so on (7). In addition, aortic medial abnormality may be present, which become increasingly important as age progresses and patients become prone to the development of ascending aorta aneurysms. The adult complex COA is commonly associated with the acquired malformations: aortic valve disease; subvalvular aortic stenosis; mitral valve disease; and CAD (8). Fifty percent of patients with untreated COA will die by the age of 10 years. About 25% of patients will die between the age of 10−20 years. Approximately 90% of patients will die before the age of 50 years (9).
Currently, there is no universal consensus on an optimal way to manage complex coarctation of the aorta accompanied by intracardiac abnormality. The main current approaches are anatomic repair and extra-anatomic repair, including one-stage treatment and two-stage treatment. In 1944, COA was first surgically corrected by Crawford and Nylin. They performed complete resection of the coarctation segment and end-to-end anastomosis of the aorta. Subsequently, other procedures have been described, including subclavian flap angioplasty, patch aortoplasty, and interposition grafting. These traditional anatomic approaches require extensive dissection of the aortic coarctation segment and dilation of collateral arteries, which have the potential to cause catastrophic bleeding; parenchymal lung injury; damage to the recurrent laryngeal or phrenic nerves; chylothorax; and spinal cord ischemia. Perhaps the most severe complication of COA surgery is paraplegia. The prolonged aortic cross clamp time in patients of advanced age, especially those with severe aortic calcification, significantly increases the risk of paraplegia. The fragility of this situation also significantly increases the likelihood of bleeding. It has been demonstrated that 5% to 30% of patients with previous coarctation repair have re-coarctation and require reintervention (2). Therefore simple anatomic repair is most suitable for younger patients with simple COA which is not accompanied by other intracardiac pathologies.
Except for anatomic repair, extra-anatomic approaches are currently preferred by many surgeons. This was first reported in 1975 and first applied by Dr. Edie who successfully established an ascending-to-descending aortic graft bypass via one-stage sternotomy and thoracotomy. The graft was routed anterior to the hilum of the left lung and to the left lateral aspect of the ascending aorta (10). In 1977, Dr. Wukasch and Cooley first described the ascending-to-abdominal bypass to treat COA (11). Then in 1980, Dr. Vijayanagar first reported an ascending-to-descending aortic graft bypass which was accomplished post-pericardially only by sternotomy, by which the graft was routed along the left heart margin (12). Following this, in 1983, Dr. Powell described a modification to Vijayanagar’s technique, by which the graft was routed around the right margin of the heart through the space behind the inferior vena cava and in front of the right inferior pulmonary vein. Finally the graft was anastomosed proximate to the right lateral aspect of the ascending aorta (13). The advantages of extra-anatomic techniques rely on the complete avoidance of the manipulation of the coarctation segment and collateral arteries, which dramatically reduces the possibility of spinal cord injury and decreases the risk of catastrophic hemorrhage. The surgical indications to extra-anatomic bypass mainly include (I) coarctation or re-coarctation and associated cardiac problems that require repair through median sternotomy; and (II) complex coarctation or re-coarctation, for which extra-anatomic bypass grafting was chosen because of the anticipated difficulties with direct anatomic repair (14). Therefore, the extra-anatomic approach is most suitable to adult complex COA accompanied with intracardiac abnormality.
One-stage repair or two-stage repair? Traditionally two-stage repair required two separate surgical procedures: COA correction by thoracotomy followed by intracardiac abnormality correction through median sternotomy. This approach can simplify each procedure, however it could make the cardiac hemodynamic instability worse when COA is corrected, which significantly increase the surgical risks and likelihood of mortality. In addition, the two-stage procedure not only increases the surgical trauma and prolongs the recovery time, but also increases the economic burden. One-stage repair implies simultaneous correction of COA and intracardiac abnormality, which eliminates the disadvantage of the two-stage approach and allows a concomitant procedure through the same incision. However, the one-stage approach could increase the technical difficulty of the procedure and prolong heart-lung machine dependent bypass time. As the techniques of cardioplegia protection and extracorporeal circulation are getting better, one-stage repair is considered to be a safe and effective approach.
How to correctly choose the extra-anatomic bypass pathway? The two approaches have their individual pros and cons, which are discussed below. In terms of ascending-to-descending aortic bypass grafting, there are several advantages including firstly, the fact that all of the surgical procedures can be performed by median sternotomy, with relatively less trauma. Secondly, the requirement for laparotomy is eliminated, which avoids danger to the peritoneal organs. The surgical complication of bowel obstruction and so on is eliminated. Thirdly, the graft is routed in a short course around the right side of the heart, which reduces the possibility of graft complications such as graft infection. On the other hand, there are some disadvantages. Firstly, the exposure of descending can be difficult, requiring the lifting of the heart cephalad with the assistance of the heart-lung machine. Otherwise, the hemodynamic instability is inevitable. This exposure is especially difficult in obese patients or those with barrel-shaped chests. The optimal exposure is essential and prerequisite for anastomosis. Secondly, it is crucial to make sure the anastomosis of the graft to descending aorta is accomplished without obvious bleeding, because such bleeding can be very difficult to control. Thirdly, there is a potential risk of causing esophageal injury.
As for ascending-abdominal aortic graft bypass, the advantages of this approach are firstly that the exposure of abdominal aorta is very simple and obvious. Secondly, the anastomosis of graft-to-aorta can be established more easily and without the assistance of a heart-lung machine. The hemostasis of anastomosis is easily achieved, which can also be strengthened by covering the retroperitoneum. However, there are still some disadvantages, which are firstly the big incision required by sternotomy-laparotomy and subsequently the prolonged recovery time. Secondly a longer graft is often needed, which possibly increases the graft-associated complications such as graft kinking, infection and so on. Thirdly, there is propensity for abdominal associated complications such as bowel obstruction, abdominal infection and so on (15).
The results of this retrospective review revealed that these two extra-anatomic aortic bypass grafting techniques can contribute to satisfactory clinical outcomes. Which of the extra-anatomic bypass approaches is selected depends on the patient's characteristics and the preference of surgeons. As for the younger patients, it is not recommended to apply the extra-anatomic approach because of considerations associated with their rapid growth.
It is concluded that the surgical management of patients with complex coarctation associated with cardiac disorders must be considered on an individual basis. The one-stage simultaneous extra-anatomic aortic graft bypass associated with intracardiac abnormalities repair is reported to be a safe and technically reliable surgical procedure which can produce satisfactory outcomes.
Acknowledgements
My sincere appreciation to Christopher Mercer for English proof reading and Yunran Li for diagram drawing.
Footnote
Conflict of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Nanjing Medical University Institutional Review Board (No. 2016-SR-144). A waiver of the need to obtain consent from patients was approved.
References
- Fernandez de Caleya D, Duarte J, Eguren A, et al. Combined therapy of coarctation and coronary heart disease in an adult. Thorac Cardiovasc Surg 1993;41:127-9. [Crossref] [PubMed]
- Foster ED. Reoperation for aortic coarctation. Ann Thorac Surg 1984;38:81-9. [Crossref] [PubMed]
- Sweeney MS, Walker WE, Duncan JM, et al. Reoperation for aortic coarctation: techniques, results, and indications for various approaches. Ann Thorac Surg 1985;40:46-9. [Crossref] [PubMed]
- Robicsek F, Hess PJ, Vajtai P. Ascending-distal abdominal aorta bypass for treatment of hypoplastic aortic arch and atypical coarctation in the adult. Ann Thorac Surg 1984;37:261-3. [Crossref] [PubMed]
- Report of the New England Regional Infant Cardiac Program. Pediatrics 1980;65:375-461. [PubMed]
- Toro-Salazar OH, Steinberger J, Thomas W, et al. Long-term follow-up of patients after coarctation of the aorta repair. Am J Cardiol 2002;89:541-7. [Crossref] [PubMed]
- Lacour-Gayet F, Bruniaux J, Serraf A, et al. Hypoplastic transverse arch and coarctation in neonates. Surgical reconstruction of the aortic arch: a study of sixty-six patients. J Thorac Cardiovasc Surg 1990;100:808-16. [PubMed]
- Attenhofer Jost CH, Schaff HV, Connolly HM, et al. Spectrum of reoperations after repair of aortic coarctation: importance of an individualized approach because of coexistent cardiovascular disease. Mayo Clin Proc 2002;77:646-53. [Crossref] [PubMed]
- Campbell M. Natural history of coarctation of the aorta. Br Heart J 1970;32:633-40. [Crossref] [PubMed]
- Edie RN, Janani J, Attai LA, et al. Bypass grafts for recurrent or complex coarctations of the aorta. Ann Thorac Surg 1975;20:558-66. [Crossref] [PubMed]
- Wukasch DC, Cooley DA, Sandiford FM, et al. Ascending aorta-abdominal aorta bypass: indications, technique, and report of 12 patients. Ann Thorac Surg 1977;23:442-8. [Crossref] [PubMed]
- Vijayanagar R, Natarajan P, Eckstein PF, et al. Aortic valvular insufficiency and postductal aortic coarctation in the adult. Combined surgical management through median sternotomy: a new surgical approach. J Thorac Cardiovasc Surg 1980;79:266-8. [PubMed]
- Powell WR, Adams PR, Cooley DA. Repair of coarctation of the aorta associated with intracardiac repair. Tex Heart Inst J 1983;10:409-13. [PubMed]
- Connolly HM, Schaff HV, Izhar U, et al. Posterior pericardial ascending-to-descending aortic bypass: an alternative surgical approach for complex coarctation of the aorta. Circulation 2001;104:I133-7. [Crossref] [PubMed]
- Wang R, Sun LZ, Hu XP, et al. Treatment of complex coarctation and coarctation with cardiac lesions using extra-anatomic aortic bypass. J Vasc Surg 2010;51:1203-8. [Crossref] [PubMed]