Arnold’s nerve cough reflex: evidence for chronic cough as a sensory vagal neuropathy
Introduction
Cough may arise from anywhere in the distribution of the vagus nerve. Cough arising from the ear (Arnold ear-cough reflex) is rare with only 15 cases having been previously reported. It is considered a medical curiosity, but now takes on more significance due to increasing observations that refractory chronic cough may be re-evaluated as a form of sensory neuropathy of the vagus nerve. Cough initiated by mechanical stimulation of the ear involves the integration of both ongoing airway vagal afferent nerve input with additional afferent input arising from the ear. Sensory laryngeal neuropathy (SLN) (1) and post viral vagal neuropathy (PVVN) (2,3) can occur after viral infections and are associated with chronic cough. Similarly, injury to various branches of the vagus nerve have been described as potential causes of unexplained chronic cough. Patients with these conditions frequently describe symptoms that suggest sensitization of the cough reflex and a neuropathic response. Examples are an abnormal throat sensation, for example, “tickle”, representing laryngeal paresthesia, increased cough sensitivity in response to a known tussigen, for example, smoke (hypertussia), and cough that is triggered in response to a nontussive stimulus, for example, exposure to cold air (allotussia) (4). Twenty percent to 40% of chronic cough patients do not respond to usual medical treatment; this is referred to as a refractory chronic cough with the precise aetiology and mechanism remaining a challenge to the medical community. We present two cases of sensory neuropathic chronic cough due to ear-cough reflex hypersensitivity and its successful treatment with gabapentin. The cases and their treatment response further strengthen the concept that vagal neuropathy may be an important cause of refractory chronic cough.
Case report
Case 1
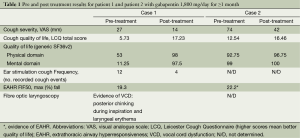
A 61-year-old female presented with non-specific chronic cough. The patient had cough duration of 30 months. She had normal spirometry and a negative response to previously trialled proton pump inhibitor and inhaled corticosteroid treatment. Associated symptoms included a scratchy, raw and very dry throat, voice changes, nocturnal cough and coughing bouts triggered by cleaning of the ears and teeth. A number of subjective and objective investigations were undertaken (Table 1). These included, cough severity by visual analogue scale (VAS), cough quality of life by Leicester Cough Questionnaire (LCQ), generic quality of life by the SF36 questionnaire, extrathoracic airway hyperresponsiveness (EAHR) (greatest fall in mid inspiratory flow during hypertonic saline challenge testing), fibre optic laryngoscopy to determine the presence of vocal cord dysfunction (VCD) and the Leicester cough monitor with external microphone to measure the frequency of the patient’s cough during ear stimulation. The patient had significantly impaired cough specific and generic quality of life, increased extrathoracic airway responsiveness, and there was evidence of VCD during fibre optic laryngoscopy, ie, paradoxical vocal fold movement with posterior chinking during inspiration (Table 1).
Full table
We stimulated the external auditory meatus with a cotton bud and this triggered a hypersensitive cough reflex with 12 discrete cough events recorded after stimulation (Figure 1).
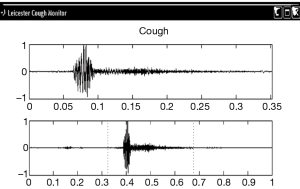
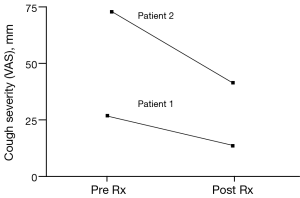
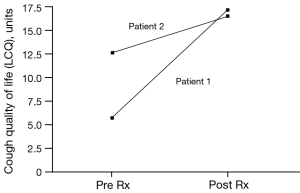
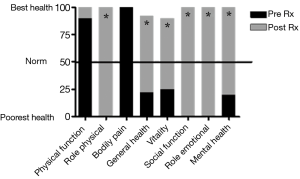
The patient was treated for sensory vagal neuropathy with gabapentin 1,800 mg/day for 1 month. The investigations were repeated and with treatment there was a significant improvement in cough severity (Table 1, Figure 2), cough quality of life (Table 1, Figure 3) and general quality of life for both the physical and mental domains (Table 1, Figure 4). Repeat stimulation of the external auditory meatus after successful gabapentin treatment (Figure 1) led to a marked reduction in cough frequency to four coughs (Table 1).
Case 2
A 69-year-old male presented with non-specific chronic cough and gave a history of cough triggered by mechanical stimulation of the ear. Patient 2 reported cough duration of 96 months and had normal spirometry and a negative response to treatment trials with inhaled corticosteroids, oral corticosteroids and nasal steroid treatment. His associated symptoms included post nasal drip syndrome, heartburn, “tickle” in throat and voice changes. Cough triggers included cold air, dry foods such as breadcrumbs, positional manoeuvres (bending down and rising after sleep) and cleaning of the ears. There was evidence of EAHR during hypertonic saline challenge testing (Table 1). The patient was treated with gabapentin 1,800 mg/day for 3 months and the investigations repeated (Table 1). There was a significant improvement in cough severity, (Table 1, Figure 2) and cough quality of life (Table 1, Figure 3) when on gabapentin 1,800 mg/day.
Written consent was obtained from patients and the study was approved by The University of Newcastle, H-2008-0241 and the Hunter New England Human Research Ethics committees, 08/03/19/3.04.
This study is registered with the Australian New Zealand Clinical Trials Register, ACTRN12608000248369.
Discussion
We describe two cases where Arnold’s nerve ear-cough reflex was a manifestation of a vagal sensory neuropathy and this was identified as the cause of a refractory chronic cough that was successfully treated with gabapentin. In both cases, the cough was triggered by mechanical stimulation of the external auditory meatus and accompanied by other neuropathic features such as throat irritation (laryngeal paresthesia), and cough triggered upon exposure to nontussive triggers such as cold air and eating (termed allotussia). These features suggest a neuropathic origin to the cough (4). We therefore used gabapentin to treat the patients based on its known success in sensory neuropathic disorders (5,6) and recently chronic cough (7,8). These observations strengthen the emerging concept that vagal sensory neuropathy may underlie many cases of refractory or idiopathic chronic cough.
SLN (1) and PVVN (2,3) have been described as potential causes of chronic cough. SLN may occur after viral infections or after mechanical trauma to the vagus or superior laryngeal nerve (1,3,9). It is thought to result in a lowered threshold for sensory laryngeal nerve firing and is consequently perceived as throat irritation and often chronic cough. In 2005, a form of hereditary sensory neuropathy was observed to be associated with chronic cough in a case study of two families (10). Affected individuals had an adult onset of paroxysmal cough, gastroesophageal reflux disease and distal sensory loss. Cough could be triggered by noxious odours or by pressure in the external auditory canal (Arnold’s ear-cough reflex). Other features included throat clearing, hoarse voice, cough syncope and sensorineural hearing loss. This disorder clearly demonstrated how cough could be linked to denervation hypersensitivity of the upper airways and oesophagus. Similarly, PVVN is a condition that occurs following an upper respiratory illness, which represents injury to various branches of the vagus nerve. The pattern of symptoms and findings in this condition are consistent with the hypothesis that viral infection causes or triggers vagal dysfunction (9). These patients may also have airway hyperresponsiveness persisting beyond the acute upper respiratory tract infection that manifests as a decrease in cough threshold in response to irritating chemical or mechanical stimuli.
There is therefore a body of evidence that links chronic cough to a neuropathic disorder involving the vagus nerve. The cases reported here extend this data by objectively documenting the Arnold nerve cough reflex, and showing that a treatment approach based on a neuropathic disorder can effectively improve cough severity, cough frequency and quality of life. We also observed an association with VCD in the first case. Cough is not uncommon in VCD, and may be a manifestation of upper airway hypersensitivity (11). Further, EAHR was also evident in one of the two cases reported. This may be a physiological example of paradoxical vocal cord closure, and is a more prevalent syndrome than first thought, potentially affecting areas under vagal innervation. In a study by Cho et al. (12) cough sensitivity was found to be closely related with EAHR during capsaicin provocation in some CC subjects. It is therefore possible that EAHR may be one of the mechanisms developing some subtypes of CC. The presence of EAHR is confirmed in this case report by hypertonic saline challenge testing with a greater than 20% fall in mid-inspiratory airflow and/or the identification of associated VCD confirmed by fibre optic laryngoscopy which also presents with extrathoracic obstruction and chronic cough.
Conclusions
This case report highlights the need for thorough investigation into patients previously diagnosed with refractory chronic cough. Uncommon causes and novel therapeutic management should be considered. In this case series chronic cough was associated with sensory neuropathy and we have shown this by identifying and triggering cough by external auditory meatus stimulation. In both cases, the cough was successfully treated with gabapentin.
Acknowledgements
Nicole M Ryan was funded by an NHMRC CCRE in Respiratory & Sleep Medicine PhD scholarship and HMRI PhD Support Scholarship donated by the Greaves Family. Peter G Gibson is an NHMRC practitioner research fellow.
Disclosure: The authors declare no conflict of interest.
References
- Lee B, Woo P. Chronic cough as a sign of laryngeal sensory neuropathy: diagnosis and treatment. Ann Otol Rhinol Laryngol 2005;114:253-7. [PubMed]
- Altman KW, Simpson CB, Amin MR, et al. Cough and paradoxical vocal fold motion. Otolaryngol Head Neck Surg 2002;127:501-11. [PubMed]
- Bastian RW, Vaidya AM, Delsupehe KG. Sensory neuropathic cough: a common and treatable cause of chronic cough. Otolaryngol Head Neck Surg 2006;135:17-21. [PubMed]
- Vertigan AE, Gibson PG. Chronic refractory cough as a sensory neuropathy: evidence from a reinterpretation of cough triggers. J Voice 2011;25:596-601. [PubMed]
- Backonja M, Glanzman RL. Gabapentin dosing for neuropathic pain: evidence from randomized, placebo-controlled clinical trials. Clin Ther 2003;25:81-104. [PubMed]
- Gilron I. Gabapentin and pregabalin for chronic neuropathic and early postsurgical pain: current evidence and future directions. Curr Opin Anaesthesiol 2007;20:456-72. [PubMed]
- Ryan NM, Birring SS, Gibson PG. Gabapentin for refractory chronic cough: a randomised, double-blind, placebo-controlled trial. Lancet 2012;380:1583-9. [PubMed]
- Van de Kerkhove C, Goeminne PC, Van Bleyenbergh P, et al. A cohort description and analysis of the effect of gabapentin on idiopathic cough. Cough 2012;8:9. [PubMed]
- Amin MR, Koufman JA. Vagal neuropathy after upper respiratory infection: a viral etiology? Am J Otolaryngol 2001;22:251-6. [PubMed]
- Spring PJ, Kok C, Nicholson GA, et al. Autosomal dominant hereditary sensory neuropathy with chronic cough and gastro-oesophageal reflux: clinical features in two families linked to chromosome 3p22-p24. Brain 2005;128:2797-810. [PubMed]
- Ryan NM, Gibson PG. Characterization of laryngeal dysfunction in chronic persistent cough. Laryngoscope 2009;119:640-5. [PubMed]
- Cho YS, Lee CK, Yoo B, et al. Cough sensitivity and extrathoracic airway responsiveness to inhaled capsaicin in chronic cough patients. J Korean Med Sci 2002;17:616-20. [PubMed]


