
Epidermal growth factor receptor inhibitors in adjuvant treatment of lung cancer—the more specific, the better?
The search and optimization for adjuvant medical treatment options beyond the surgical removal of the primary tumor, is of paramount importance to reduce the high risk of recurrence and death in lung cancer patients.
According to the recently published study entitled “Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II–IIIA (N1–N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): a randomised, open-label, phase 3 study”, the adjuvant administration of the epidermal growth factor receptor (EGFR) inhibitor gefitinib was superior to vinorelbine combined with cisplatin in patients with operated EGFR-mutant non-small cell lung cancer (NSCLC) (1).
In more detail, the authors enrolled patients between the age of 18–75 years with completely resected (R0) stage II–IIIA (N1–N2) EGFR-mutant (exon 19 deletion or exon 21 Leu858Arg substitutions) NSCLC. Patients were randomized into either treatment with the first-generation EGFR inhibitor gefitinib (250 mg once daily) for 24 months or intravenous vinorelbine (25 mg/m2 on days 1 and 8) in addition to intravenous cisplatin (75 mg/m2 on day 1) every 3 weeks for overall four cycles (1). Twenty-seven centers in China participated in this open-label phase III trial. The primary endpoint was disease-free survival (DFS).
Previous data has shown that only 20–25% of patients diagnosed with NSCLC are suitable for surgical resection with curative intention at the time point of diagnosis (2). Adjuvant chemotherapy has been shown to improve survival in patients with early-stage NSCLC (3). Vinorelbine in combination with cisplatin is the recommended standard-of-care adjuvant treatment for stage IIA–IIIB resected NSCLC, independent of EGFR mutation status. The combination vinorelbine/cisplatin has led to a small but statistically significant increase in overall survival and DFS (4-6). Still, the 5-year survival in patients with stage II–IIIA NSCLC is poor with only 14–30% (7). Thus, further testing of novel treatment modalities is warranted to improve the patients’ outcome in future.
The primary endpoint in the study by Zhong et al. was DFS assessed by the investigators, defined as time from randomization to documented disease relapse, or death (1). Secondary endpoints were overall-survival (time from randomization to death from any cause), 3-year DFS, 5-year DFS, as well as 5-year overall survival. The authors also evaluated safety and tolerability, and the patients’ quality of life. An intention-to-treat population was defined which comprised all randomized patients, and additionally the authors outlined a modified intention-to-treat population comprising randomized patients who received at least one dosage of study medication without any major protocol deviations (1).
Overall, 222 patients were randomized, 111 to gefitinib and 111 to vinorelbine plus cisplatin. It was found that median DFS was significantly longer with gefitinib (28.7 months; 95% CI: 24.9–32.5) as compared to vinorelbine combined with cisplatin (18 months; 95% CI: 13.6–22.3). The most common adverse event, grade III or worse, in the gefitinib group were elevated alanine aminotransferase and aspartate aminotransferase. Two patients reported this event vs. no patients with the chemotherapy regimen was diagnosed with this side effect. In the vinorelbine and cisplatin group the most frequent grade III or worse adverse events were neutropenia (30 patients), leucopenia (14 patients) and vomiting (8 patients).
The authors concluded that adjuvant gefitinib led to a significantly longer DFS as compared to treatment with vinorelbine and cisplatin in patients with completely resected stage II–IIIA (N1–N2) EGFR-mutant NSCLC. Since there is superior DFS, reduced toxicity and improved quality of life, adjuvant gefitinib may serve as a standard-of-care option for adjuvant treatment instead of chemotherapy (1).
Previous clinical trials mainly focused on the metastatic disease setting. It has been shown that administration of EGFR-TKIs significantly prolongs progression-free survival (PFS) as compared to platinum-based regimens in EGFR-mutant NSCLC (8,9). In a study by Maemondo and colleagues, 230 patients with EGFR-mutant NSCLC who had received no previous chemotherapy, were randomly assigned to either gefitinib or carboplatin-paclitaxel treatment (8). PFS was significantly longer in the gefitinib group as compared to the standard-chemotherapy group (median PFS 10.8 vs. 5.4 months; P<0.001), which resulted in early termination of this study. Response rates were also higher in the gefitinib group (73.7% vs. 30.7%; P<0.001). However, in this study one patient who had received gefitinib died from interstitial lung disease (8). Zhang et al. published a meta-analysis in 2018, searching multiple databases with respect to gefitinib and erlotinib as treatment for NSCLC (10). Overall, 40 studies comprising 9,376 participants were analyzed. Both gefitinib and erlotinib proved effective for advanced NSCLC, with comparable PFS and overall survival. For erlotinib, dose reduction had to be carried out more frequently and grade III–V adverse events were reported more often as well. Thus, the authors of this meta-analysis concluded that gefitinib might be the better treatment for advanced NSCLC, since tolerability was superior to erlotinib, and antitumor effectiveness was equal, whilst fewer adverse events were reported (10). Given that viewpoint, the choice to select gefitinib in the adjuvant setting in clinical routine makes sense.
Though the results of the improvement of DFS in the study by Zhong and colleagues are remarkable, one should consider the limitations of this study, especially in consideration of the clinical relevance and economical costs. First of all, when administering TKIs, it is mandatory to do close follow-up investigations of the patients for early detection of adverse events (11). Each EGFR-TKI has characteristic adverse events, primarily diarrhea, skin disorders and liver dysfunction. In 2018, Kimura et al. published a study for the evaluation of cost-effectiveness of the TKIs gefitinib, afatinib and erlotinib in patients with NSCLC harboring EGFR mutations (11). Forty-one patients with a median age of 64 years (afatinib), 71 years (gefitinib) and 69 years (erlotinib), were evaluated retrospectively. Gefitinib was orally administered at 250 mg once a day. The cost-data revealed that gefitinib was the most inexpensive treatment (11). The cost-effectiveness analysis showed the lowest costs per median survival time for gefitinib, while erlotinib was most expensive. In the same study, adverse events were also analyzed. For gefitinib, rash (64.3%), raises in aspartate aminotransferase (57.1%), anaemia (42.9%), and diarrhea (35.7%) were observed. Neither in the study by Kimura et al., nor in the study by Zhong et al., treatment-related deaths for gefitinib were reported (1,11). Costs probably will decrease significantly as gefitinib patent protection ends soon and generic drugs are on their way to approval.
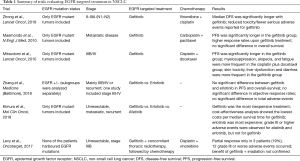
However, not all data about gefitinib is promising. In 2017, a study on gefitinib in combination with irradiation followed by chemotherapy in patients with inoperable stage III NSCLC was carried out (12). In this phase II study, patients were administered gefitinib 250 mg once daily, with concomitant thoracic radiotherapy, followed by chemotherapy (intravenous cisplatin combined with vinorelbine). The regimen was carried out as first-line treatment in a population of unselected stage IIIB NSCLC patients. None of the patients harbored EGFR-mutations. Four weeks after radiotherapy, partial response was only observed in 3 patients (19%). For 6 patients (38%) stable disease was reported, and 7 patients (44%) progressed (12). 12 grade III or worse adverse events occurred, and one individual died because of pneumonitis. The main side effects were gastrointestinal (81%), skin disorders (81%), general (56%) or respiratory (50%). As a conclusion of this analysis, the benefit of gefitinib combined with radiotherapy could not be confirmed (12). Nevertheless, it has to be kept in mind that none of the participants in the above-mentioned study had a positive EGFR mutation status. Further limitations of the study are the lack of generalizability from the Asian population to Caucasians, the question whether next generation EGFR-inhibitors such as afatinib or osimertinib would do even better, and if the DFS advantage will be transferred to an overall survival benefit. A molecular screening process and selection in the adjuvant setting will be as important as in the metastatic situation. This should include ALK-aberrations, ROS and BRAF mutations and PD-L1 expression status, to test the most powerful and approved drugs already available in the metastatic setting. Importantly, as PD-1/PD-L1 inhibitors do not work in EGFR-mutated patients in the metastatic setting, these screening processes should also include adjuvant studies of immune checkpoint inhibitors. Table 1 summarizes all the above-mentioned trials for a quick review.
Full table
Clearly, further studies with larger patient cohorts in a non-Asian population are warranted to assess effectiveness, but also safety, of gefitinib and the next generation EGFR-inhibitors. A closer look has to be taken at overall survival, since most studies focused only on PFS. Some studies only found a significant advantage for the EGFR-inhibitor with regards to PFS, but no difference in overall survival whatsoever. It is questionable, for how long response to treatment is observed, and when resistance to EGFR-targeted treatment manifests. A side effect of EGRF TKIs that is fortunately very rare, but often lethal, is interstitial lung disease. A few cases of patients having died of interstitial lung disease or pneumonitis as a side effect of EGFR TKIs, have been reported. Future research needs to focus on how to manage this side effect, and what alternative drug can be used to continue treatment. When analyzing the effect of EGFR TKIs, it is generally imperative to always separate patients with positive EGFR mutation status from those without EGFR mutations, to avoid this confounder. Summarizing the already existing data, we believe that treatment with EGFR-inhibitors is considerably safe and effective in advanced NSCLC, especially in tumors harboring EGFR mutations.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): a randomised, open-label, phase 3 study. Lancet Oncol 2018;19:139-48. [Crossref] [PubMed]
- Arriagada R, Dunant A, Pignon JP, et al. Long-term results of the international adjuvant lung cancer trial evaluating adjuvant Cisplatin-based chemotherapy in resected lung cancer. J Clin Oncol 2010;28:35-42. [Crossref] [PubMed]
- Burdett S, Pignon JP, Tierney J, et al. Adjuvant chemotherapy for resected early-stage non-small cell lung cancer. Cochrane Database Syst Rev 2015.CD011430. [PubMed]
- Eberhardt WE, De Ruysscher D, Weder W, et al. 2nd ESMO Consensus Conference in Lung Cancer: locally advanced stage III non-small-cell lung cancer. Ann Oncol 2015;26:1573-88. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Akerley W, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 4.2016. J Natl Compr Canc Netw 2016;14:255-64. [Crossref] [PubMed]
- Kris MG, Gaspar LE, Chaft JE, et al. Adjuvant Systemic Therapy and Adjuvant Radiation Therapy for Stage I to IIIA Completely Resected Non-Small-Cell Lung Cancers: American Society of Clinical Oncology/Cancer Care Ontario Clinical Practice Guideline Update. J Clin Oncol 2017;35:2960-74. [Crossref] [PubMed]
- American Cancer Society. Non-small cell lung cancer survival rates, by stage. 2016. Available online: https://www.cancer.org/cancer/non-small-cell-lung-cancer/detection-diagnosis-staging/survival-rates.html
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [Crossref] [PubMed]
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [Crossref] [PubMed]
- Zhang W, Wei Y, Yu D, et al. Gefitinib provides similar effectiveness and improved safety than erlotinib for east Asian populations with advanced non-small cell lung cancer: a meta-analysis. BMC Cancer 2018;18:780. [Crossref] [PubMed]
- Kimura M, Yasue F, Usami E, et al. Cost-effectiveness and safety of the molecular targeted drugs afatinib, gefitinib and erlotinib as first-line treatments for patients with advanced EGFR mutation-positive non-small-cell lung cancer. Mol Clin Oncol 2018;9:201-6. [PubMed]
- Levy A, Bardet E, Lacas B, et al. A phase II open-label multicenter study of gefitinib in combination with irradiation followed by chemotherapy in patients with inoperable stage III non-small cell lung cancer. Oncotarget 2017;8:15924-33. [Crossref] [PubMed]


