A comparison of ketamine-midazolam and ketamine-propofol combinations used for sedation in the endobronchial ultrasound-guided transbronchial needle aspiration: a prospective, single-blind, randomized study
Introduction
Endobronchial ultrasound guided transbronchial needle aspiration (EBUS-TBNA) is an ultrasonography method which is developed for imaging neighboring tissues next to the airways. It has been used as a first choice for either diagnosis of mediastinal and hilar lymphadenopathies or staging of lung cancer since it is a minimally invasive method with high diagnostic value (1).
However, EBUS-TBNA can cause anxiety in patients, and can lead to hemodynamic instability, in addition to this, it can affect negatively the performance of the bronchoscopist and the comfort of patients. The anxiety of patients is relieved by sedation. A successful sedation protects reflexes, enables patients to follow instructions and provides a comfortable work area for the bronchoscopist (2).
Sedation level ranges from awake to general anesthesia status depending on the dose of agent. Conscious sedation is mostly preferred for procedures that require fast recovery (3). Midazolam and propofol are commonly used for sedation in all endoscopic procedures. Midazolam, a benzodiazepine, leads to anxiolysis, anterograde amnesia and light hypnosis. It is preferred for its highly amnestic property (4,5). Meanwhile, propofol has both amnestic and antiemetic effects. Propofol is chosen for its rapid onset time and short acting effect as well as for its quick recovery (6). However, both agents don’t have analgesic effect. Ketamine, causes fast deep sedation and analgesia, protects respiration and airway reflexes when it is given slowly. It increases heart rate (HR) and blood pressure slightly and causes bronchodilatation. It is a good choice in sedation of the patients with airway sensitivity. On the other hand, ketamine can cause laryngospasm by increasing secretions (7-9). The other side effects such as nausea, vomiting, hallucinations and anxiety may occur by using ketamine alone (7-10).
We think that the sedation used for interventional bronchoscopy is a unique procedure. Moreover, the sedative agents should also have exceptional advantages without having severe side effects. Until now, there is no certain sedation regimen or ideal agent for EBUS-TBNA. We hypothesized that usage of the sedative agents’ combination can alleviate this problem.
The addition of ketamine may reduce hypoventilation and dose dependent side effects in patients having procedures under sedation with midazolam or propofol (8-11). Ketamine and propofol in the same injector (ketofol) is used successfully for sedation in emergency department (8,12). To our knowledge, we did not encounter any study showing that ketofol is used to induce sedation for bronchoscopic procedures in the literature. A combination of ketamine and midazolam may result in fewer side effects and shorter recovery time (9). These combinations are supported since each agent balances others hemodynamic and respiratory side effects (9,13,14).
We designed this study to evaluate the clinical efficacy and safety of two different sedative agents (midazolam and propofol) combined with ketamine during conscious sedation for EBUS-TBNA. For this aim, we compared respiratory and hemodynamic effects, agent consumptions, sedation scores, recovery time, side effects, and the satisfaction of the patients as well as the bronchoscopist.
Materials and methods
This prospective study was conducted with the approval of the ethic committee and with the written informed consent of the patients. Sixty patients between 18-70 years old, with American Society of Anesthesiology (ASA) classification of I-II, and without contraindication for conscious sedation were included in this trial. Patients who had allergy against study agents, ischemic heart disease, high level of kidney and liver function tests, electrolyte imbalance, mental disease, central nervous system disease, drug or alcohol dependency, upper respiratory system infection, glaucoma, and porphyria were excluded from the study.
Performance of EBUS
Patients were informed and asked to sign consent forms 24 h before the procedure. Patients were premedicated with 0.5 mg (intramuscular, im) atropine 30 min before the procedure. In the operation room, electrocardiogram (EKG), non-invasive blood pressure, and peripheral oxygen saturation (SpO2) were monitorized and a peripheral intravenous (iv) cannula was placed. Five minutes (min) before the procedure had started, 2% lidocaine spray was pumped ten times (1 pump =10 mg lidocaine) to the pharenx. A convex probe-EBUS (BF-UC 180F, Olympus, Tokyo, Japan) was used to examine the lymph nodes, and the ultrasound images were processed with a dedicated scanner (EU-ME1, Olympus, Tokyo, Japan). The bronchoscopist used 22-gauge needle to sample the lymph nodes and applied 2% lidocain while the bronchoscope was passing through vocal cords and carina as well as bronches. Total topical lidocaine dose was limited to 200 mg. All patients received 4 L/dk oxygen via nasal canula during the procedure. The oxygen level was increased to 6 L/dk when SpO2 was less than 90%.
Design of study
Patients were randomly divided into two groups by sealed envelope method. The patients in Group 1 were first given 0.05 mg/kg (iv) midazolam and then 2 min later, given 0.25 mg/kg ketamine (iv). The patients in Group 2 received ketofol (mixture of 1:1 ratio ketamin- propofol). Ketofol was prepared by combining ketamine 1 mL (50 mg/mL), propofol 5 mL (10 mg/mL), and saline 4 mL in a single syringe. 1 mL of ketofol includes ketamine 5 mg and propofol 5 mg. Group 2 first received 0.25 mg/kg (for each agent, ketamine and propofol) and then 2 min later it was repeated as 0.125 mg/kg. Sedation level was evaluated using Ramsay sedation score (RSS) during the procedure. According to this score: 1, patient anxious, agitated; 2, patient co-operative, orientated; 3, patient responds to verbal stimulation only; 4, patient asleep, rapid response to light stimulation or loud auditory stimulus; 5, patient asleep, slow response to light stimulation or loud auditory stimulus; 6, no response to any stimulation. For both groups, when RSS was 3, it allowed the fiberoptic bronchoscope to pass vocal cords (15). When RSS was 2, additional doses (0.25 mg/kg ketamine in Group 1 and 0.125 mg/ kg ketamine-propofol mixture in Group 2) were given to the patients to maintain sedation.
Mean arterial blood pressure (MABP), HR, SpO2, respiratory rate (RR), RSS, coughing severity, and doses of the medications were the recorded parameters. These parameters were recorded on the following times: in the beginning, before any sedative was given (T1); 2 min after first sedation (T2) as well as 5 min (T3), 10 min (T4), 15 min (T5), 20 min (T6), 25 min (T7), 30 min (T8), 35 min (T9), 40 min (T10), 45 min (T11), and 50 min (T12).
Severity of cough was evaluated using a three-point scale during the procedure (16). The scaling was as follows: 1, single coughing; 2, more than one episodes of coughing; 3, severe sustained coughing. Complications and side effects were also recorded during the procedure. We used Modified Aldrete Score (MAS) to evaluate recovery (17). Recovery time was determined as the time until MAS 9.
We did not inform the bronchoscopist and the patients about the sedative agents used during the procedure. We asked and assessed the bronchoscopist’s satisfaction with the sedation. The answers were: 0, not enough; 1, moderate; 2, good; 3, excellent. Two hours after the procedure, we asked the patients following questions to understand the satisfaction of the patients with the sedation:
- Whether he/she remember the EBUS-TBNA procedure.
- No
- Partially
- Yes
- In general, are you satisfied with the sedation during the procedure?
- Yes, I am
- No, I am not
- Would you let the same sedatives to be used for the next time?
- Yes
- Whether he/she remember the EBUS-TBNA procedure.
- No
- Partially
- Yes
- In general, are you satisfied with the sedation during the procedure?
- Yes, I am
- No, I am not
- Would you let the same sedatives to be used for the next time?
- Yes
- No
Statistical analysis
All the data were analyzed using “SPSS for Windows 16” program. For non-quantitative data, tables were made and differential analysis between groups was evaluated using chi-square. The independent-sample t-test was used for regularly distributed data and the Mann Whitney U test was used for irregularly distributed data to compare groups. To evaluate differences in the groups, we used repeated measurement variance analysis test for regularly distributed data. For irregularly distributed data, we used the Wilcoxon test to evaluate differences in the groups. Correlation was evaluated using correlations test. P<0.05 was accepted as statistically significant. The data were combined at the times 15-20 as 20 min; 25-30 as 30 min; 35-40 as 40 min; 45-50 as 50 min. The recovery time at 35 and 60 in values was combined as 30 min.
Results
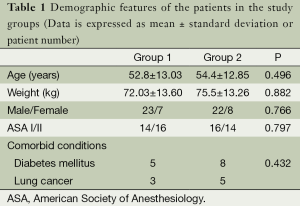
Sixty patients were included and analyzed in this study (Figure 1). Table 1 shows demographic data, ASA and comorbid conditions of two groups. There was no statistical difference between two groups for these parameters (P>0.05).
Full table
Median RSS values were found between 2 and 3 in both groups. Number of patients whose RSS was 3 at T9 (35th min of induction) was higher in Group 1 compared to Group 2 (P<0.05). There was no difference between two groups for RSS values in the other periods (P>0.05).
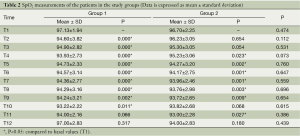
Mean SpO2 values are shown in Table 2. There was no statistical difference in SpO2 between two groups (P>0.05). When SpO2 values are compared to the beginning, SpO2 decreased significantly at T2, T3, T4, T5, T6, T7, T8, T9 and T10 in Group 1 (P<0.05). Similarly, significant decrease in SpO2 level compared to the beginning was detected in Group 2 at T4, T5, T6, T7, T8, T9 and T11 (P<0.05). Mean SpO2 values were not below 90% in both groups during the study.
Full table
When RR values were compared, there was no statistical significant difference between two groups (P>0.05). In Group 1, RR showed a significant increase compared to the beginning, only at T2 (P<0.05). Whereas, there was a significant decrease in RR compared to the beginning level at T8 in Group 2 (P<0.05). Respiratory depression (<10 respiration/min) was not observed in both groups.
When we compared MABP values, there was no statistically significant difference between two groups (Figure 2) (P>0.05). MABP significantly increased compared to the beginning level T2, T4, T5 and T6 after induction in both groups (P<0.05). When HR values were compared between two groups, at T4, there was a significant increase in Group 1 (Figure 3, P<0.05). There was no significant difference in HR at the other recording times (P>0.05). In Group 1 and 2, HR increased significantly at the times T2, T3, T4, T5, T6, T7, T8, and T9. In Group 2, HR also increased significantly at T10 (P<0.05).
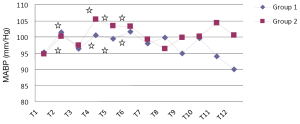
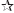 , P<0.05: compared to basal values (T1). MABP, mean arterial blood pressure.
, P<0.05: compared to basal values (T1). MABP, mean arterial blood pressure.
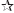 , P<0.05: compared to basal values (T1).
, P<0.05: compared to basal values (T1).  , P<0.05: comparison between the groups.
, P<0.05: comparison between the groups.The coughing scores are shown in Table 3. There was no significant difference between two groups for these scores (P>0.05). The mean EBUS-TBNA procedure time was 34±7.81 min in Group 1 and it was 33.67±9.64 min in Group 2. The procedure time was similar between two groups (P>0.05). When two groups were compared according to the side effects, there was no statistical difference between two groups (P>0.05). The characteristics of the patients with complications are shown in Table 4. While there was no side effects in Group 1, nausea (n=1), hallucination (n=1), and ventricular extrasystole (n=1) were observed in Group 2. Throughout the study, we did not observe life threatening complication due to EBUS-TBNA procedure or the sedation method.
Full table

Full table
Mean recovery times were 27.67±4.09 and 25.00±7.31 min in Group 1 and Group 2, respectively. The recovery time was significantly longer in Group 1 when compared to Group 2 (P<0.05).
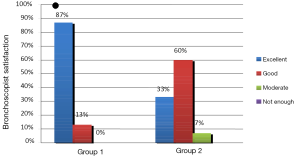
In the evaluation of the satisfaction of the bronchoscopist with the sedation, Group 1 had significantly more excellent score compared with Group 2 (Figure 4, P<0.05). Groups were similar related to remembering the procedure, patient satisfaction, and given permission for the next time to use the same sedative agents (Table 5, P>0.05).
 , P<0.05: comparison between the groups.
, P<0.05: comparison between the groups.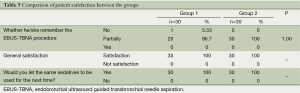
Full table
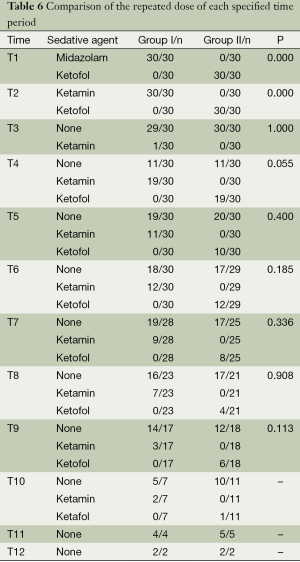
Consumed mean ketamine was similar in two groups (55.7±15.0 mg in Group 1 and 50.6±13.72 mg in Group 2) (P>0.05). Additionally, in Group 1, 3.6±0.7 mg midazolam and in Group 2, 50.6±13.72 mg propofol were used. The repeated doses of each specified time period of the groups was shown in the Table 6.
Full table
Additional doses were needed for all cases in both groups. The median repeated dose number was 2 (range, 1-8) in Group 1 and 2 (range, 1-4) in Group 2. There was no significant difference in repeated dose number between the groups (P=0.480). The repeated dose number was positively correlated with the body weight both in Group 1 (r=0.308; P=0.098) and Group 2 (r=0.169; P=0.371) without statistical significance.
Discussion
In our study, we compared ketamine-midazolam and ketamine-propofol combinations used for conscious sedation in EBUS-TBNA procedure. We demonstrated that both combinations are similarly effective and safe. In the present study, ketamine’s combination with either midazolam or propofol provided good levels of satisfaction for the patients and the bronchoscopist without remarkable side effects.
Kennedy et al. compared general anesthesia and sedation for EBUS-TBNA procedure in the patients with lung cancer (18). They found that sedation is as much comfortable as general anesthesia and it has more advantages regarding recovery time and hospital discharge compared to general anesthesia. Anxiety during the local procedures can stimulate sympathetic system and can cause hypertension, arrhythmia, and increase in myocardial oxygen consumption. Sedative agents are used to relieve anxiety and its’ negative effects (19-21). However, some investigators still think that bronchoscopic and endoscopic procedures can be performed without sedation (22,23). They consider that using sedation increases risk of respiratory depression due to combination of different agents, decreases patient’s cooperation during the procedure, and causes an increase in both recovery time and the cost of hospitalization (22-25). In a study evaluating FOB with sedation, it was reported that major complication ratio was 0.08-5%. Moreover, half of these complications were due to the sedation itself (26).
For this reason, anesthetists should evaluate consciousness level carefully. Sedation scores are used to assess consciousness level subjectively, while “bispectral index” (BIS), which measures direct effect of sedation on brain, is used for objective evaluation (27,28). BIS monitoring did not discriminate mild-to-moderate sedation or moderate-to-deep sedation, as measured by the RSS for the patients undergoing procedural sedation (28). There is nothing yet that measures sedation depth quantitatively that can replace the qualitative assessment for procedural sedation (29) Thus, we preferred RSS for the assessment of consciousness level.
The patients should be monitored carefully because of the cardiovascular and respiratory side effects of both local anesthesia and sedation (30). For these reasons, blood pressure, ECG, SpO2 and RR should be recorded. Routine oxygen support is suggested to prevent hypoxia during fiberoptic bronchoscopy (FOB) under local anesthesia (31). The most important complications are respiratory depression and desaturation owing to the sedative agent (32). We applied continuous nasal oxygen and SpO2 monitorization both in the operation room and in the recovery room to prevent desaturation due to EBUS-TBNA.
Midazolam and propofol are commonly used for sedation either solely or in combination with other agents (32-35). They can depress cardiovascular and respiratory system depending on the dosage (33-37). The usage of propofol or midazolam for sedation in bronchoscopic procedures can cause severe side effects like hypoxemia, tachycardia, and hypotension (32,35,37).
Even though ketamine protects laryngeal reflexes, its use in adults is limited since it causes increase in HR, hypertension, nausea, vomiting, hallucinations, and anxiety (8,38-40). Ketamine’s effects are balanced with combination of midazolam and propofol. Hwang et al. showed that patient controlled sedation with ketamine in combination with propofol during FOB provides hemodynamic stability and high patient satisfaction (14). Willman et al. reported hypoxia, without the requirement of intubation, in only 3 out of 114 patients receiving ketofol for sedation and analgesia in emergency room (8). Akin et al. reported that fewer numbers of patients needed additional dose in ketamine and propofol group than only propofol group in their study performed on pediatric patients (41). While none of the patients suffered from apnea or desaturation in ketamine and propofol group, they observed apnea in six patients and desaturation in four patients in propofol group.
Chudnofsky et al. observed apnea in three patients and laryngospasm in one patient in their study performed on 70 adult patients injected with ketamine-midazolam combination for painful procedures (9). In another study, Drummod studied the effects of ketamine and midazolam on airway muscle activities and found that while 10 of 12 patients given midazolam had airway obstruction and respiratory distress, none of the 11 patients injected with ketamine had any respiratory problem (42). In our study, there was no difference between groups regarding to SpO2 and RR and we did not observe any respiratory depression in our patients.
Willman et al. encountered treatment required hypertension and hallucination in three patients in their study (8). Additionally given midazolam did not cause hypotension, vomiting, and any respiratory distress that required endotracheal intubation. In our study, although both groups were hemodynamically stable, we observed a temporary increase in MABP, and HR when bronchoscope passes vocal cords. It was not clinically significant increase and we think that it can be prevented by increasing topical anesthetic dose.
Laryngospasm, airway obstruction, apnea, a rise in blood pressure and myocardial oxygen demand can be observed as side effects of ketamine (43). During EBUS-TBNA or any other airway procedures, coughing and increase in secretion can happen due to both ketamine and procedure by itself (44). We observed increase in coughing score as during bronchoscope passes the vocal cords in both groups despite sufficient level of sedation. The secretion increase caused by ketamine was not clinically too important and it was tolerated by frequent aspiration.
Mortero et al. showed that coadminastration of ketamine attenuates propofol-induced hypoventilation and may provide earlier recovery of cognition (13). In their another study, Akin et al. concluded that adding low dose ketamine to propofol did not increase recovery time in 60 patients (ages 1 month-13 year old) who underwent cardiac catheterization (45). Willman et al. showed that median recovery time was 15 min (range, 5-45 min) for ketafol (8). Chudnofsky et al. founded that mean recovery time for ketamine-midazolam combination as 64±24 min because of higher dose usage (9). The recovery time was approximately 25 min with lower doses in the present study. We attributed that longer recovery time in Group 1 was due to higher RSS at 35th min compared to Group 2 without clinical significance.
Adequate sedation reduces patient anxiety and improves tolerance and satisfaction with the procedure. Putinati et al. showed that patient tolerance improved with conscious sedation during FOB without any increased cardiorespiratory risks (19). They found that doctors’ satisfaction score is much higher than patients’ satisfaction score. They concluded that doctors cannot exactly evaluate patients’ responses. Willman et al. reported that both patient’s and doctor’s satisfaction with ketamine-propofol combination were very well (8). Steinfort et al. showed that EBUS-TBNA may be safely performed with very high patient satisfaction under conscious sedation (34). Khajavi et al. showed that ketamine and propofol combination have more patients satisfaction than fentanil and propofol combinations (46). In our study, sedation was not evaluated as “not enough” for any patient by doctors. Even though, “excellent” response was significantly higher than Group 2, “good and excellent” response was 100% in Group 1 and 93% in Group 2. In general, all of the patients were satisfied. Both groups were similar regarding to patient and doctor satisfaction.
Our study had some limitations. The present study evaluating sedation for EBUS-TBNA was performed in a single center. A multicenter study is required to reveal more objective results and evaluate the clinical efficacy and safety of two different sedative regimens in future. The use of end tidal CO2 (ETCO2) or transcutaneous CO2 monitoring would provide additional safety to gauge efficient ventilation in sedation regimens (29). Although we didn’t investigate the cost-effectiveness of the agents, the assessment of the cost-effectiveness could increase the quality of study as well as the efficacy and the safety.
Conclusions
Until now, there is no ideal sedation agent without undesirable effects. The sedative agent should be chosen according to procedure, the age of patient, general health condition, and the experience of bronchoscopist and anesthesiologist. Still there is no sufficient study about the safe sedative agent for EBUS-TBNA. Our study showed that both combinations were adequate and effective to relieve anxiety and to depress hypertension and tachycardia. Besides, there were no side effects like hypoxia, and hypotension indicating that the doses were safe. In conclusion, both ketamine-midazolam and ketamine-propofol combinations in the doses we used provide safe, effective, as well as comfortable conscious sedation for EBUS-TBNA and there was no superiority between two combinations. We think that ketamine’s combination with either midazolam or propofol provided good levels of satisfaction for the patients and the bronchoscopist without remarkable side effects.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Annema JT, Versteegh MI, Veseliç M, et al. Endoscopic ultrasound-guided fine-needle aspiration in the diagnosis and staging of lung cancer and its impact on surgical staging. J Clin Oncol 2005;23:8357-61. [PubMed]
- Rolo R, Mota PC, Coelho F, et al. Sedation with midazolam in flexible bronchoscopy: a prospective study. Rev Port Pneumol 2012;18:226-32. [PubMed]
- Whitwam JG, McCloy RF. Principles and Practice of Sedation, 2nd ed. Oxford: Blackwell Scientific,1998:1-54.
- Friedman AG, Mulhern RK, Fairclough D, et al. Midazolam premedication for pediatric bone marrow aspiration and lumbar puncture. Med Pediatr Oncol 1991;19:499-504. [PubMed]
- Broennle AM, Cohen DE. Pediatric anesthesia and sedation. Curr Opin Pediatr 1993;5:310-4. [PubMed]
- Kennedy RM, Luhmann JD, Luhmann SJ. Emergency department management of pain and anxiety related to orthopedic fracture care: a guide to analgesic techniques and procedural sedation in children. Paediatr Drugs 2004;6:11-31. [PubMed]
- Krauss B, Brustowicz RM. eds. Pediatric Procedural Sedation and Analgesia. Baltimore: Lippincott, Williams &Wilkins, 1999:97-103.
- Willman EV, Andolfatto G. A prospective evaluation of “ketofol” (ketamine/propofol combination) for procedural sedation and analgesia in the emergency department. Ann Emerg Med 2007;49:23-30. [PubMed]
- Chudnofsky CR, Weber JE, Stoyanoff PJ, et al. A Combination of Midazolam and Ketamine for Procedural Sedation and Analgesia in Adult Emergency Department Patients. Acad Emerg Med 2000;7:228-35. [PubMed]
- Nejati A, Moharari RS, Ashraf H, et al. Ketamine/propofol versus midazolam/fentanyl for procedural sedation and analgesia in the emergency department: a randomized, prospective, double-blind trial. Acad Emerg Med 2011;18:800-6. [PubMed]
- De Oliveira GS Jr, Fitzgerald PC, Hansen N, et al. The effect of ketamine on hypoventilation during deep sedation with midazolam and propofol: A randomised, double-blind, placebo-controlled trial. Eur J Anaesthesiol 2013. [Epub ahead of print]. [PubMed]
- Andolfatto G, Willman E. A prospective case series of single-syringe ketamine-propofol (Ketofol) for emergency department procedural sedation and analgesia in adults. Acad Emerg Med 2011;18:237-45. [PubMed]
- Mortero RF, Clark LD, Tolan MM, et al. The effects of small-dose ketamine on propofol sedation: respiration, postoperative mood, perception, cognition, and pain. Anesth Analg 2001;92:1465-9. [PubMed]
- Hwang J, Jeon Y, Park HP, et al. Comparison of alfetanil and ketamine in combination with propofol for patient-controlled sedation during fiberoptic bronchoscopy. Acta Anaesthesiol Scand 2005;49:1334-8. [PubMed]
- Ramsay MA, Savege TM, Simpson BR, et al. Controlled Sedation with Alphaxalone-Alphadolone. Br Med J 1974;2:656-9. [PubMed]
- Minogue SC, Ralph J, Lampa MJ. Laryngotracheal topicalization with lidocaine before intubation decreases the incidence of coughing on emergence from general anesthesia. Anesth Analg 2004;99:1253-7. [PubMed]
- Aldrete JA. The post-anesthesia recovery score revisited. J Clin Anesth 1995;7:89-91. [PubMed]
- Kennedy MP, Shweihat Y, Sarkiss M, et al. Complete mediastinal and hilar lymph node staging of primary lung cancer by endobronchial ultrasound: moderate sedation or general anesthesia? Chest 2008;134:1350-1. [PubMed]
- Putinati S, Ballerin L, Corbetta L, et al. Patient satisfaction with conscious sedation for bronchoscopy. Chest 1999;115:1437-40. [PubMed]
- Stolz D, Chhajed PN, Leuppi JD, et al. Cough suppression during flexible bronchoscopy using combined sedation with midazolam and hydrocodone: a randomised, double blind, placebo controlled trial. Thorax 2004;59:773-6. [PubMed]
- Smith I, Avramov MN, White PF. A comparison of propofol and remifentanil during monitored anesthesia care. J Clin Anesth 1997;9:148-54. [PubMed]
- Colt HG, Morris JF. Fiberoptic bronchoscopy without premedication. A retrospective study. Chest 1990;98:1327-30. [PubMed]
- Al-Atrakchi HA. Upper gastrointestinal endoscopy without sedation: a prospective study of 2000 examinations. Gastrointest Endosc 1989;35:79-81. [PubMed]
- Gonzalez R, De-La-Rosa-Ramirez I, Maldonado-Hernandez A, et al. Should patients undergoing a bronchoscopy be sedated? Acta Anaesthesiol Scand 2003;47:411-5. [PubMed]
- Du Rand IA, Blaikley J, Booton R, et al. British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults: accredited by NICE. Thorax 2013;68:i1-i44. [PubMed]
- Dreisin RB, Albert RK, Talley PA, et al. Flexible fiberoptic bronchoscopy in the teaching hospital: yield and complications. Chest 1978;74:144-9. [PubMed]
- Innes G, Murphy M, Nijssen-Jordan C, et al. Procedural sedation and analgesia in the emergency department. Canadian Consensus Guidelines. J Emerg Med 1999;17:145-56. [PubMed]
- Gill M, Green SM, Krauss B. A study of the Bispectral Index Monitor during procedural sedation and analgesia in the emergency department. Ann Emerg Med 2003;41:234-41. [PubMed]
- Bahn EL, Holt KR. Procedural sedation and analgesia: a review and new concepts. Emerg Med Clin North Am 2005;23:503-17. [PubMed]
- Kallio H, Rosenberg PH. Advances in ophthalmic regional anaesthesia. Best Pract Res Clin Anaesthesiol 2005;19:215-27. [PubMed]
- Milman N, Faurschou P, Grode G, et al. Pulse oximetry during fibreoptic bronchoscopy in local anaesthesia: frequency of hypoxaemia and effect of oxygen supplementation. Respiration 1994;61:342-7. [PubMed]
- Stolz D, Kurer G, Meyer A, et al. Propofol versus combined sedation in flexible bronchoscopy: a randomised non-inferiority trial. Eur Respir J 2009;34:1024-30. [PubMed]
- Wright SW, Chudnofsky CR, Dronen SC, et al. Midazolam use in the emergency department. Am J Emerg Med 1990;8:97-100. [PubMed]
- Steinfort DP, Irving LB. Patient satisfaction during endobronchial ultrasound-guided transbronchial needle aspiration performed under conscious sedation. Respir Care 2010;55:702-6. [PubMed]
- Clark G, Licker M, Younossian AB, et al. Titrated sedation with propofol or midazolam for flexible bronchoscopy: a randomised trial. Eur Respir J 2009;34:1277-83. [PubMed]
- Jensen JT, Banning AM, Clementsen P, et al. Nurse administered propofol sedation for pulmonary endoscopies requires a specific protocol. Dan Med J 2012;59:A4467. [PubMed]
- Oztürk T, Cakan A, Gülerçe G, et al. Sedation for fiberoptic bronchoscopy: fewer adverse cardiovascular effects with propofol than with midazolam. Anasthesiol Intensivmed Notfallmed Schmerzther 2004;39:597-602. [PubMed]
- White PF, Way WL, Trevor AJ. Ketamine--its pharmacology and therapeutic uses. Anesthesiology 1982;56:119-36. [PubMed]
- Green SM, Krauss B. The semantics of ketamine. Ann Emerg Med 2000;36:480-2. [PubMed]
- Song JW, Shim JK, Song Y, et al. Effect of ketamine as an adjunct to intravenous patient-controlled analgesia, in patients at high risk of postoperative nausea and vomiting undergoing lumbar spinal surgery. Br J Anaesth 2013;111:630-5. [PubMed]
- Akin A, Esmaoglu A, Tosun Z, et al. Comparison of propofol with propofol-ketamine combination in pediatric patients undergoing auditory brainstem response testing. Int J Pediatr Otorhinolaryngol 2005;69:1541-5. [PubMed]
- Drummond GB. Comparison of sedation with midazolam and ketamine: effects on airway muscle activity. Br J Anaesth 1996;76:663-7. [PubMed]
- Strayer RJ, Nelson LS. Adverse events associated with ketamine for procedural sedation in adults. Am J Emerg Med 2008;26:985-1028. [PubMed]
- Heinz P, Geelhoed GC, Wee C, et al. Is atropine needed with ketamine sedation? A prospective, randomised, double blind study. Emerg Med J 2006;23:206-9. [PubMed]
- Akin A, Esmaoglu A, Guler G, et al. Propofol and Propofol-Ketamine in Pediatric Patients Undergoing Cardiac Catheterization. Pediatr Cardiol 2005;26:553-7. [PubMed]
- Khajavi M, Emami A, Etezadi F, et al. Conscious Sedation and Analgesia in Colonoscopy: Ketamine/Propofol Combination has Superior Patient Satisfaction Versus Fentanyl/Propofol. Anesth Pain Med 2013;3:208-13. [PubMed]