Patient preference for a maintenance inhaler in chronic obstructive pulmonary disease: a comparison of Breezhaler and Respimat
Introduction
Chronic obstructive pulmonary disease (COPD) affects quality of life (1,2) and is associated with a high level of mortality (3) and significant financial burden (4). A report by WHO (3) predicted that by 2030 COPD will be the third leading cause of death globally. However, in a recent report (5) the National Center for Health Statistics in the United States revealed that COPD had already become the third leading cause of death in the United States by 2011. A separate report (6) by the Centers for Disease Control and Prevention published in 2015 indicated that the total financial burden of COPD in USA alone in 2010 was $36 billion and predicted that it would rise to $49 billion by 2020.
The control of COPD depends on the availability of effective medications and on good compliance which has been described as ‘of paramount importance’ (7) in reducing the occurrence of acute exacerbations, hospitalisations and mortality as well as improving quality of life (8). Although a number of effective medications are available, several studies have confirmed that long-term compliance is low (9-11) and that this can affect the quality of life, worsen patient outcomes and increase health care costs (12). Interestingly, whilst the ATS/ERS guidelines (13) discuss the use of the various medications, they do not cover the subject of compliance.
Reduced compliance is often associated with difficulties in the physical handling of an inhaler (14-16) particularly among patients of advancing age and those with cognitive impairment (17,18) which can worsen as the disease progresses (19). Poor inhalation technique may further reduce clinical effectiveness by reducing the amount of drug deposited in the lungs (16). Conversely, good inhalation technique ‘is the cornerstone’ of COPD management and is associated with improved control (20).
Despite the recommendations of both the Global Initiative for Chronic Obstructive Lung Disease (GOLD) (21) and The National Institute for Health and Care Excellence (NICE) (22) in the United Kingdom that training in the use of inhalers is vital there is ample evidence that it is often absent or at least, infrequent (23,24). In this scenario, ‘ease of use’ assumes even greater significance if the patient is to reap the benefits of full compliance with dosage instructions.
Against this background of a predicted increase in the prevalence of COPD and the potential benefits associated with maintenance inhalers that are easy to use, a research study was undertaken to compare two maintenance inhalers.
The study was designed to answer three key questions:
- Which maintenance inhaler handling-related attributes are perceived as most important by participants?
- Which inhaler is perceived by participants to be superior against a number of these handling related attributes?
- Which inhaler is preferred overall?
Methods
Unlike clinical trials that are designed to measure specific outcomes such as the effectiveness of a drug in conjunction with a medical device used in combination products, primary market research is based on observation and opinion. Therefore, as a primary non-interventional research study, ethics approval was not required in accordance with ESOMAR code of conduct (https://www.esomar.org/uploads/public/knowledge-and-standards/codes-and-guidelines/ICCESOMAR_Code_English.pdf). In addition, all patients provided oral informed consent at the beginning of recruitment and written informed consent before the start of the actual interview.
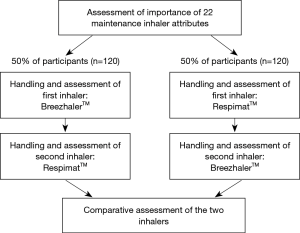
The study was conducted in four countries among 240 maintenance inhaler-naive participants. The original intention was for the study population to be split equally between participants with and without COPD. However, for reasons explained in detail under Characteristics of the study population, it was felt justified to recruit ‘consumers with a high risk of developing COPD’ instead of maintenance inhaler-naive COPD patients in two countries. Moreover, to avoid introducing bias in the study, none of the study population had had previous experience of either of the inhalers or indeed with any other maintenance inhaler.
All participants were provided with two inhalers and depending on the inhaler, a blister containing a capsule or a cartridge. Neither inhaler contained active ingredients. The inhalers were BreezhalerTM (BH—available in combination with Ultibro, Seebri and Onbrez from Novartis) and RespimatTM (RM—available in combination with Spiriva, Spiolto and Striverdi from Boehringer Ingelheim) (25). Throughout the research all individual inhalers were ‘single blinded’ by the addition of a black box sticker over the brand names and referred to by the moderators as ‘inhaler Blue’ (BH) and ‘inhaler Green’ (RM) respectively. The research was structured as follows:
In addition to designing the questionnaires and overall management of the study, GfK was responsible for all data tabulations and analyses. The responsibility for the interpretation of these data was shared by the five authors of the study.
‘Warm up’
The moderators confirmed that the study would take about 45 minutes to complete and briefly described its purpose. They also confirmed that the proceedings would be tape recorded but re-assured participants of complete confidentiality.
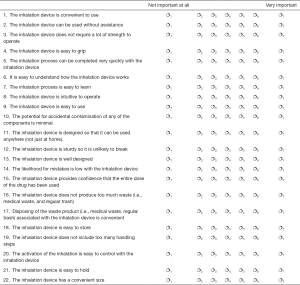
Participants were then asked to fill out a self-completion sheet (Supplementary file 1) in which they were asked ‘please indicate how important it is for you personally that an inhalation device would offer each of the attributes listed below assuming that it would be used according to its respective dosage instructions’. Each attribute was to be rated on a 7-point scale where 1= ‘not at all important’ and where 7= ‘very important’.
The ranking of the attributes was based on the percentage of participants who rated them either 6 or 7 on the rating scale (Top 2 box score). It was considered that this approach reflected a positive and unequivocal response by the participants to the question. The Top 2 box scores were later aggregated and enabled analysts to develop a rank order of all 22 attributes in terms of how important participants perceived it to be that a maintenance inhaler should offer each attribute.
The list of attributes was developed following extensive research of published work including papers on the relationship between handling difficulties with maintenance inhalers and subsequent reductions in compliance.
The handling process
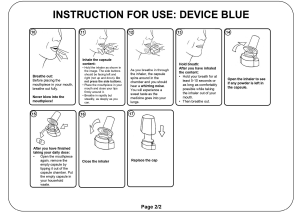
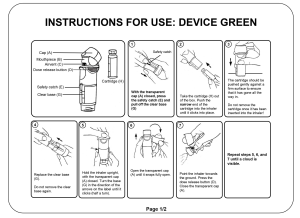
Participants handled each inhaler in a random order and on three separate occasions. Immediately prior to the second handling, participants were provided with the respective ‘Instructions for use’ (IFU) which was equivalent to that included in the packaging leaflet of the commercial product. However, in order to minimise the impact of the IFUs on inhaler preference per se, they were both provided in black and white with illustrations rather than photographs (Supplementary files 2 and 3).
Non-comparative assessment
Following the third handling of each inhaler participants completed a second self-completion sheet where they were asked to what extent they agreed that the inhaler offered each of the 22 attributes on the sheet (Supplementary file 4) using a 7 point rating scale where 1 = ‘I do not agree at all’ and 7= ‘I completely agree’. The Top 2 box scores were aggregated for each attribute as before.
The handling process and the non-comparative assessment were then repeated for the second inhaler.
Comparative assessment
The final stage of the study involved two direct comparisons of the inhalers. In the first of these, participants allocated 100 points between the inhalers where the greater the preference, the higher the share of points. When aggregated, the results established an overall preference for one inhaler over the other. In the second comparison participants allocated 100 points between each of the inhalers on six specific attributes:
- Ease of use;
- Confidence that a full dose has been taken;
- Intuitiveness;
- Speed of preparation;
- Ease of learning how to handle the inhaler;
- Design.
Study population
A total of 240 maintenance inhaler-naive participants split equally across Australia, Brazil, Germany and Japan completed the research. A co-diagnosis of COPD and asthma was an exclusion criterion in the COPD group. Asthma was also an exclusion criterion in the ‘device/disease-naive consumer’ group since patients with this condition might have had experience of rescue therapy using an inhaler.
The study comprised 240 participants all of whom were ‘maintenance inhaler-naive’ at the time of their interview in order to exclude any prior experience of operating a maintenance inhaler which might otherwise have biased the results. Originally, it was intended that half of the country sample had to have been free of any long-term disease that could have required the use of a maintenance inhaler of any sort—so called ‘device/disease-naive consumers’. The other half had to have had a physician’s diagnosis of COPD but no experience with a maintenance inhaler. However, it proved impractical to recruit this type of COPD patient in Germany and Japan because the vast majority of newly-diagnosed COPD patients in these two countries are started on long-term maintenance treatment including a maintenance inhaler immediately following diagnosis. In fact, just three were recruited in Germany and none in Japan. It was decided that in these two countries only a suitable alternative would be to recruit consumers who were at a ‘high risk’ of developing COPD based on a COPD population screener (26) but who were also maintenance inhaler-naive. It was felt that this was an acceptable deviation from the original planned population since like the other two types of participants none of this latter group would have had any prior experience of using a maintenance inhaler. A detailed breakdown of the numbers by country of the three groups of participants is available in Table 1.
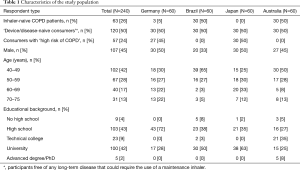
Full table
Statistical methods
Paired t-test (27).
In order to quantify the difference between the inhalers in a score or rating such as for example, ‘intuitiveness of device’, a paired t-test methodology was applied where a P value of <0.05 was regarded as statistically significant at the 95% confidence level.
Results
Rank order of importance of attributes
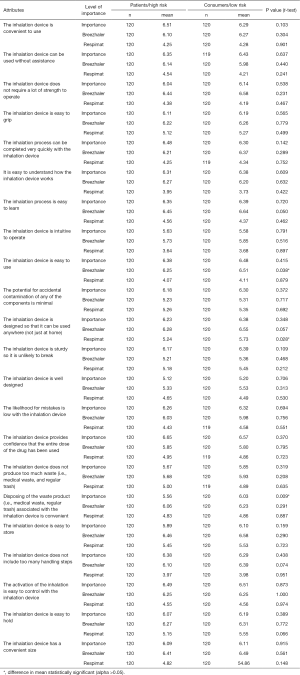
The consolidation of responses to the first task in the ‘warm up’ involved the rating of the importance to participants of 22 handling-related attributes (Figure 1). Participants rated ‘provides confidence that a full dose of the drug has been taken’ as the most important of all 22 attributes with 92% awarding it a Top 2 box score (either 6 or 7). Following closely were a number of attributes associated with ‘easiness’ including ‘activation is easy to control’ (88%), ‘process is easy to learn’ (87%) and ‘easy to use’ (87%). Other high scoring attributes included ‘low likelihood to make mistakes (84%) and ‘easy to understand how device works’ (83%). This latter was the only key attribute to show a significant difference across the participating countries where significantly fewer participants in Japan (68%) gave it a Top 2 box score compared with participants in the other three countries (Germany 92%, Brazil 88% and Australia 85%).
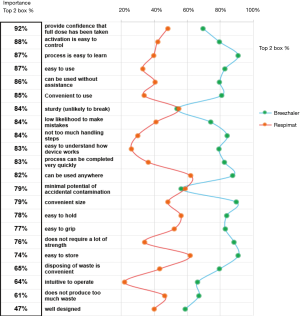
Non-comparative assessment of performance of the inhalers against attributes
Following the three handlings of the individual inhaler, participants were asked to indicate on self-completion sheet 2 (Supplementary file 4) their responses to the question ‘indicate how much you agree that this inhalation device reflects those statements [attributes] for you personally’. Figure 1 shows that participants judged BH to be superior to RM on all but 2 of the 22 attributes (‘sturdy/unlikely to break’ and ‘minimal potential of accidental contamination’) where there was no statistically significant difference between the two inhalers (P value for all ratings =0.000).
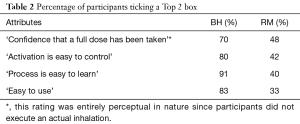
Given the importance to compliance of full dosage and overall ‘easiness’ of use, Table 2 reveals the extent of the superiority of BH over RM on the top four rated attributes.
Full table
There were four attributes that could also be gathered under the heading ‘easiness’ in addition to those featured in Table 2—‘easy to understand how device works’, ‘easy to hold’, ‘easy to grip’ and ‘easy to store’—on all of which BH was statistically superior to RM. The results for attributes that could be described as ‘convenience-related’ were similarly in favour of BH. Moreover, BH was rated significantly higher on other attributes such as ‘not too many handling steps’, ‘low likelihood to make mistakes’ and ‘process can be completed very quickly’. (P value for all ratings =0.000).
Additional analyses conducted to compare the responses of the COPD patient/high risk groups with those of the ‘device/disease naive’ (low risk) group revealed that there were significant differences in only 3 of the 22 attributes—‘the inhalation device is easy to use’ (BH), ‘the inhalation device is designed so that it can be used anywhere not just at home (RM)’ and ‘disposing of the waste product (i.e., medical waste, regular trash) associated with the inhalation device is convenient’ (overall rating). Detailed findings of these analyses are available in Supplementary file 5. However, these differences between the populations although statistically significant, were minor and did not alter the general tendency towards BH being perceived to be superior to RM.
Comparative assessment of the inhalers
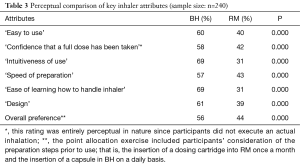
In the final phase of the study participants were asked to perform ‘point allocation’ exercises on six key attributes and on their overall preference for one inhaler over the other. In these exercises 100 points were shared between the two inhalers. Table 3 reveals that overall a significant majority of participants considered BH to be superior on all six attributes and on ‘overall preference’. (All differences are highly significant at a 95% confidence level).
Full table
There were a small number of statistically significant differences across the four participating countries regarding the attributes in Table 3. Statistically fewer participants in Brazil considered BH to be superior to RM on ‘easy to use’ and ‘overall preference’ compared with participants in Germany and significantly fewer participants in Australia considered BH to be superior to RM on ‘confidence that a full dose has been taken’ compared with participants in either Germany or Japan. There were no significant differences across the countries regarding ‘speed of preparation’, ‘ease of learning how to handle the inhaler’ and ‘design’.
Additional analyses revealed no significant differences in the responses of the COPD patients/high risk groups and the ‘device/disease naive (low risk) group regarding the key attributes shown in Table 3 and in overall preference.
Discussion
Chronic obstructive pulmonary disease is responsible for significant levels of morbidity (28), mortality (3) and healthcare and wider economic costs (4). In the absence of medications that can reverse the inevitable decline in lung function, management guidelines (21,22) state that the goals of treatment of COPD are a reduction in the rate of long-term decline in lung function, prevention and treatment of exacerbations, reduction in hospitalizations and mortality, relief of disabling dyspnoea and improvement of exercise tolerance and health-related quality of life (29,30).
Although a wide range of medications is available to help achieve these goals, their efficacy depends to a large extent on the handling characteristics of the individual maintenance inhaler. Therefore, a maintenance inhaler with superior handling as judged against a number of handling-related attributes could provide improved compliance, improved overall disease control and improved clinical outcomes.
With this background in mind, a research study was designed to answer three key questions; which maintenance inhaler handling-related attributes were perceived as most important by participants, which inhaler was perceived by participants to be superior against a number of handling-related attributes and which inhaler was preferred overall?
A crucial aspect of clinical efficacy and therefore long-term control is the amount of active drug reaching the lungs. Critically, the participants in this study rated ‘provides confidence that a full dose has been taken’ as the most important of the 22 attributes listed. When asked to what extent they personally considered that the individual inhaler offered this attribute in a non-comparative assessment of the two inhalers, a significant majority (70%) considered that BH did offer this attribute compared with less than half (48%) for RM.
There is extensive evidence that training in the handling of maintenance inhalers is far from universal (23,24) in which case it can be argued that the more intuitive and easier to handle an inhaler is, the more likely it will be that patients will comply with dosage instructions and so benefit from improved disease control, clinical outcomes and quality of life.
Among the 22 attributes against which participants rated the inhalers were ‘intuitiveness to use’ and seven that could be grouped under the heading ‘easiness’ (‘easy to use’, ‘activation easy to control’, ‘process easy to learn’, ‘easy to understand how device works’, ‘easy to hold’, ‘easy to grip’ and ‘easy to store’). In the non-comparative assessment a significantly greater percentage of participants considered that BH would provide each of these attributes compared to RM. It follows that the more ‘intuitive’ an inhaler is to use, the less time will be spent on learning the process and the more likely it will be that the patient uses the inhaler correctly.
Several other attributes that could be defined as ‘convenience-related’ such as ‘convenient to use’ and ‘convenient size’ and others such as ‘not too many handling steps’, ‘low likelihood to make mistakes’ and ‘process can be completed very quickly’ were also included in the rating exercise and again, BH was judged to be significantly superior to RM.
Furthermore, in a direct comparison BH was rated significantly higher than RM on six specific attributes, ‘confidence that a full dose has been taken’ (58% vs. 42%), ‘easy to use’ (60% vs. 40%), ‘intuitiveness of use’ (69% vs. 31%), ‘speed of preparation’ (74% vs. 26%), ‘ease of learning how to handle’ (77% vs. 23%) and ‘design’ (64% vs. 36%).
Finally, in a direct choice between the inhalers, a significant majority of participants preferred BH to RM overall (56% vs. 44%).
Limitations of research
The nature of primary market research (PMR) meant that participants did not perform the inhalation step.
The sample of 240 participants may not be a true reflection of ‘real life’. That said, results did reveal considerable commonality across all four countries on many of the attributes assessed in the research.
Conclusions
Compliance with recommended dosage regimens is crucial for the maintenance of disease control and improved clinical outcomes in COPD. In the absence of a cure for COPD, prevalence is predicted to increase year by year and so it will be increasingly important in the future that maintenance inhalers are as simple to handle as possible in order to optimise compliance, to improve clinical outcomes and therefore to contain the burden of COPD.
Results of this study showed that Breezhaler was rated higher by significantly greater numbers of participants in its ability to deliver against 20 of 22 maintenance inhaler handling-related attributes including ‘confidence that a full dose has been taken’ which participants judged to be the most important of all 22 attributes. Such confidence is of profound importance considering the clinical benefits derived from the delivery of a full dose of active drug to the lungs. The delivery of a full dose of drug could have additional benefits by decreasing the risk of under- or overdosing that some patients might face due to the uncertainty with their current inhaler.
Other inhaler-related attributes where Breezhaler was rated superior to Respimat included ‘intuitiveness to use’ and several associated with ‘easiness’ including ’easy to use’ and ‘easy to learn the process’. These results are also of considerable importance given the lack of training in the use of maintenance inhalers where evidence confirms the relationship between handling difficulties and reduced clinical effectiveness. In this regard, the easier an inhaler is to use, the more likely the patient will comply with dosage recommendations.
In summary, the combination of superiority on the vast majority of maintenance inhaler handling-related attributes particularly ‘confidence that a full dose has been taken’ and several associated with ‘easiness’ and its status as the preferred inhaler suggests that Breezhaler offers an opportunity for improved compliance, improved disease control and most importantly, improved clinical outcomes in COPD.
Supplementary
Supplementary file 1
Self-completion sheet 1
For each of these statements please indicate how important it is for you personally that an inhalation device would offer each of the attributes listed below. We would like you to think about what might be important to you if you were asked to use an inhalation device in the future. For the assessment please use a 7-point scale, 1 meaning “Not Important at All” and 7 meaning “Very Important”.
Supplementary file 2: IFU Breezhaler
Supplementary file 3: IFU Respimat
Supplementary file 4
Self-completion Sheet 2 Blue
For each of these statements below, please indicate how much you agree that this inhalation device reflects those statements for you personally. For the assessment please use a 7-point scale, 1 meaning “I do not agree at all” and 7 meaning “I completely agree”.
Supplementary file 5
Acknowledgements
The research was designed and executed by GfK Switzerland AG, Basel and sponsored by Novartis Pharma AG, Basel.
Footnote
Conflicts of Interest: P O’Hagan is an independent healthcare consultant and medical writer and was commissioned and remunerated by GfK Switzerland AG to support data interpretation and manuscript development. J Dederichs is an employee of Novartis Pharma AG, Basel. B Viswanad is an employee of Novartis Healthcare, Hyderabad, India. M Gasser and S Walda are employees of GfK Switzerland AG, Basel.
Ethical Statement: As a primary non-interventional research study, ethics approval was not required in accordance with ESOMAR code of conduct (https://www.esomar.org/uploads/public/knowledge-and-standards/codes-and-guidelines/ICCESOMAR_Code_English.pdf). In addition, all patients provided oral informed consent at the beginning of recruitment and written informed consent before the start of the actual interview.
References
- Partridge MR, Karlsson N, Small I. Patient insight into the impact of chronic obstructive pulmonary disease in the morning: an internet research. Curr Med Res Opin 2009;25:2043-8. [Crossref] [PubMed]
- O’Hagan P, Chavannes N. The impact of morning symptoms on daily activities in chronic obstructive pulmonary disease. Curr Med Res Opin 2014;30:301-14. [Crossref] [PubMed]
- Burden of COPD. Available online: (last accessed 1st Dec 2015).http://www.who.int/respiratory/copd/burden/en
- Chapman KR, Mannino DM, Soriano JB, et al. Epidemiology and costs of chronic obstructive pulmonary disease. In: Rabe KF, Doriano JB. editors. Series: “The Global Burden of Chronic Obstructive Pulmonary Disease” Eur Resp J 2006;27:188-207.
- Hoyert DL, Xu J. Deaths: Preliminary Data for 2011. National Vital Statistics Reports 2012;61. [PubMed]
- Ford ES, Murphy LB, Khavjou O, et al. Total and State-Specific Medical and Absenteeism Costs of COPD Among Adults Aged => 18 Years in the United States for 2010 and Projections through 2020. Chest 2015;147:31-45. [Crossref] [PubMed]
- Restrepo RD, Alvarez MT, Wittnebel LD, et al. Medication adherence issues in patients treated for COPD. Int J Chron Obstruct Pulmon Dis 2008;3:371-84. [Crossref] [PubMed]
- Sin DD, McAlister FA, Man SF, et al. Contemporary management of chronic obstructive pulmonary disease: scientific review. JAMA 2003;290:2301-12. [Crossref] [PubMed]
- World Health Organization. Adherence to long-term therapies: policy for action. Meeting report 4–5 June 2001. Available online: www.who.int/chp/knowledge/publications/adherencerep.pdf
- Corden ZM, Bosley CM, et al. Home nebulized therapy for patients with COPD: patient compliance with treatment and its relation to quality of life. Chest 1997;112:1278-82. [Crossref] [PubMed]
- Turner J, Wright E, Mendella L, et al. Predictors of patient adherence to long-term home nebulizer therapy for COPD. The IPPB Research Group. Intermittent Positive Pressure Breathing. Chest 1995;108:394-400. [Crossref] [PubMed]
- DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care 2004;42:200-9. [Crossref] [PubMed]
- Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med 2011;155:179-91. [Crossref] [PubMed]
- Hesselink AE, Penninx BW, Wijnhoven HA, et al. Determinants of an incorrect inhalation technique in patients with asthma or COPD. Scand J Prim Health Care 2001;19:255-60. [Crossref] [PubMed]
- Lenney J, Innes J, Crompton G. Inappropriate inhaler use: assessment of use and patient preference of seven inhalation devices. Respir Med 2000;94:496-500. [Crossref] [PubMed]
- Lavorini F, Magnan A, Dubus J, et al. Effect of incorrect use of dry powder inhalers on management of patients with asthma and COPD. Respir Med 2008;102:593-604. [Crossref] [PubMed]
- Bourbeau J, Bartlett S. Review. Patient adherence in COPD. Thorax 2008;63:831-8. [Crossref] [PubMed]
- Barrons R, Pegram A, Borries A. Inhaler device selection: special considerations in elderly patients with chronic obstructive pulmonary disease. Am J Health Syst Pharm 2011;68:1221-32. [Crossref] [PubMed]
- Schou L, Ostergaard B, Rasmussen L, et al. Cognitive dysfunction in patients with chronic obstructive pulmonary disease – A systematic review. Respir Med 2012;106:1071-81. [Crossref] [PubMed]
- Capstick T and Clifton I. From Expert Review of Respiratory Medicine. Inhalation technique and Training in People with Chronic Obstructive Pulmonary Disease and Asthma. Medscape 27th Jan 2012.
- Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2013. Available online: (last accessed 15th Nov 2015).http://www.goldcopd.org
- National Institute for Health and Clinical Excellence (NICE). Chronic obstructive pulmonary disease: national clinical guidance for management of chronic obstructive pulmonary disease in adults in primary and secondary care. Update CG101, published June 2010 of Guideline 12. Thorax 2004;59:1-232.
- Broeders ME, Vincken W, Corbetta L. The ADMIT series – Issues in Inhalation Therapy. 7. Ways to improve pharmacological management of COPD: the importance of inhaler choice and inhalation technique. Prim Care Respir J 2011;20:338-43. [Crossref] [PubMed]
- Ho SF. Inhalation technique in older people in the community. Age Ageing 2004;33:185-8. [Crossref] [PubMed]
- Montuschi P, Ciabattoni G. Bronchodilating drugs for chronic obstructive pulmonary disease: current status and future trends. J Med Chem 2015;58:4131-64. [Crossref] [PubMed]
- Martinez FJ, Raczek AE, Seifer FD, et al. Development and initial validation of a self-scored COPD Population Screener questionnaire (COPD-PS). COPD 2008;5:85-95. [Crossref] [PubMed]
- Altman D. Practical Statistics for Medical Research. London: Chapman and Hall, 1991.
- Montuschi P. Editorial (Hot Topic: Drugs for chronic obstructive pulmonary disease). Curr Med Chem 2013;20:1461-3. [Crossref] [PubMed]
- Montuschi P, Macagno F, Valente S, et al. Inhaled muscarinic acetylcholine receptor antagonists for treatment of COPD. Curr Med Chem 2013;20:1464-76. [Crossref] [PubMed]
- Fuso L, Mores N, Valente S, et al. Long-acting beta-agonists and their association with inhaled corticosteroids in COPD. Curr Med Chem 2013;20:1477-95. [Crossref] [PubMed]