Predictors and impact of right heart failure severity following left ventricular assist device implantation
Introduction
Right heart failure (RHF) is a common complication following left ventricular assist device (LVAD) placement, with prevalence reports ranging between 10–50% of patients (1-4). Left ventricular (LV) contractile forces contribute 20–40% of right ventricular (RV) output (4), and the hemodynamic effects of LVAD placement can have deleterious effects on native RV function (5). Modifying RV physiology by altering preload and afterload of the LV, optimizing RV protection, and minimizing blood transfusions intra-operatively have been shown to limit both RHF occurrence and progression. RHF following LVAD implantation, even when treated medically and with right ventricular assist device (RVAD) support, has been associated with increased hospital length of stay (LOS) and decreased survival of patients, even after successful cardiac transplantation (1,6-9). Although multiple risk factors for predicting RHF following LVAD implantation are available (10-12), the optimal method to anticipate this complication remains uncertain (13). Furthermore, the methods used for the diagnosis and categorization of RHF following LVAD are debated. Most definitions are based on a combination of hemodynamic derangement indicators and the duration of postoperative inotropes (1). This article aims to further delineate the contributing preoperative and intraoperative risk factors for RHF development following LVAD implantation and to demonstrate the effect of RHF on patients’ postoperative outcomes.
Methods
Clinical
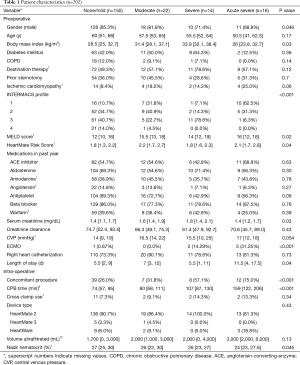
We performed a retrospective analysis using a prospectively maintained database of consecutive LVAD patients implanted between 2008 and 2016 at the Baylor University Medical Center, Dallas, Texas. In this cohort, RHF was categorized using the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) inotrope-based definition (14) of none, mild (≤7 days on inotropes), moderate (8–14 days), severe (>14 days), and acute-severe (requiring RVAD). Preoperative characteristics evaluated included gender, age, body mass index (BMI), INTERMACS profile, and other medical comorbidities (Table 1). Perioperative data included cross-clamp use, cardiopulmonary bypass (CPB) time, nadir hematocrit, and volume removed via ultrafiltration during bypass. The primary outcome was severity of RHF. Other post-operative variables ranged from infectious processes, hemodynamic values, device problems, and death at 1 year. The clinical course for each patient was followed for 1 year following LVAD implantation. The protocol for data collection was approved by the Institutional Review Board of Baylor University Medical Center Dallas (IRB File #011-274), and informed consent was waived.
Full table
Statistical analysis
The Kruskal Wallis test and the Cochran-Armitage trend test (or Fisher’s Exact test, as needed) were used to examine differences in patient characteristics and surgical outcomes across the categories of RHF. We built a multivariable logistic regression model via stepwise selection, in which we considered all variables having a significant association in univariate analyses for the dichotomized outcome of moderate or worse RHF compared to no RHF (moderate, severe, and acute severe categories were combined for this analysis due to limited sample size; there were no instances of mild RHF). We performed a competing risks analysis using the Fine and Gray method to assess the effect of RHF severity on survival, while accounting for the competing risk of transplantation (15). For simplicity, we use the terms ‘survival’ and ‘mortality’ in this manuscript when we truly mean transplant-free survival and transplant-free mortality. Continuous variables are reported as median [25th percentile, 75th percentile]. Categorical variables are reported as frequencies and percentages. Statistical significance is defined as a P value <0.05. Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
There were 202 subjects included in this analysis, 52 (26%) of whom developed a moderate or worse form of RHF (22 moderate, 14 severe, 16 acute severe). Approximately 90% of the subjects received a HeartMate 2 device. Age, comorbidities, and medication use were similar across RHF severity; however, gender, serum creatinine, pre-operative LOS, Model for End-Stage Liver Disease (MELD) scores, HeartMate Risk scores, and INTERMACS profiles differed significantly (Table 1). One hundred twenty-eight (85.3%) of those without RHF were male, compared to only 11 (68.8%) of those with acute-severe RHF. The median pre-operative LOS more than doubled as RHF severity increased (5.5 to 11.5 days, P=0.04). There were 10 (62.5%) INTERMACS-profile-1 patients in the acute-severe RHF group compared to 16 (10.7%), 7 (31.8%), and 1 (7.1%) patients in the no RHF, moderate, and severe groups, respectively (P<0.001). Significant differences were also observed in intra-operative variables such as CPB time and nadir hematocrit. The median CPB time ranged from 74 to 159 minutes as RHF severity worsened. Nadir hematocrit decreased from 27% to 24% as RHF severity worsened.
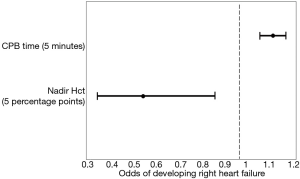
Due to the limited number of individuals who developed moderate or worse forms of RHF, we were unable to build a multivariable model to classify RHF severity; however, we combined the categories of moderate, severe, and acute severe into one category and compared it to those without RHF. The optimal model had an area under the curve of 0.77 and utilized only two variables, CPB time and nadir hematocrit. For every 5 additional minutes of CPB, the risk of developing a moderate or more severe form of RHF increased by 12% [odds ratio (OR): 1.12, 95% confidence interval (CI): 1.06–1.17]. Alternatively, for every increase of 5 percentage points of hematocrit, the risk of developing a moderate or more severe form of RHF decreased by 47% (OR: 0.53, 95% CI: 0.33–0.86) (Figure 1).
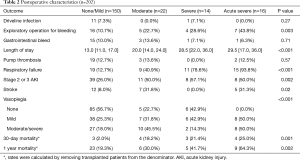
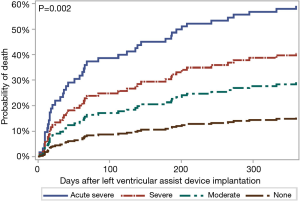
Postoperative LOS differed significantly across the RHF severity groups, with the shortest median stay (13 days) for those who did not develop RHF and the longest median stay (29.5 days) for those who developed acute-severe RHF (Table 2). The following postsurgical outcomes also differed significantly by RHF severity: incidence of stage 2/3 acute kidney injury (AKI), as defined by Kidney Disease Improving Global Outcomes (KDIGO) guidelines (16) (ranging from 26% in the no RHF group to 57% in the severe group, P=0.002), respiratory failure (12.7% to 93.8%, P<0.001), stroke (0% to 31.8%, P=0.002), exploratory operation for bleeding (10.7% to 43.8%, P=0.003), and vasoplegia severity (P<0.001) (Table 2). The rates of mortality at 30 days (range, 2.0–25%; P=0.001) and 1 year (19.3–64.3%, P=0.002) increased with RHF severity. When considered as a time-to-event outcome, those who developed acute-severe RHF were at 5.4 (95% CI: 2.5–11.8) times the risk of 1-year mortality when compared to those who did not have RHF (Figure 2). Similarly, those with severe RHF were at 3.2 (95% CI: 1.2–8.6) times the risk, and those with moderate RHF were at 2.1 (95% CI: 0.8–5.2) times the risk of 1-year mortality.
Full table
Discussion
Approximately 1 in 4 patients in this cohort developed moderate or worse RHF following LVAD implantation. Of the preoperative variables analyzed, MELD score, HeartMate Risk Score, INTERMACS profile, BMI, serum creatinine, and LOS significantly differed by RHF severity. Although there was a significant difference in preoperative serum creatinine across the RHF severity groups, preoperative estimated glomerular filtration rates, which take into account gender, age, and race, demonstrated no such difference. Intraoperatively, extended CPB time and decreased hematocrit, which has potential to necessitate blood transfusions (6), were found to be associated with increased RHF severity. Increasing rates of concomitant procedures and ECMO across RHF severity may partially explain the longer CPB times as RHF severity worsened. Following LVAD placement, RHF severity was significantly related to postoperative LOS, AKI, respiratory failure, and stroke. Finally, the presence and degree of RHF negatively impacted 30-day and 1-year survival rates post-LVAD implantation.
Our results confirm prior studies regarding the lack of a relation between RHF and patient age (6,7,10). Our work also confirms that females may be at increased risk for RHF after LVAD placement (8,17). Further, our work reiterates the link between RHF and preoperative serum creatinine (12), elevated BMI (11), and longer preoperative (10) and postoperative LOS (7). A prior study demonstrated that the need for RVAD placement during the initial LVAD procedure significantly increased the risk of RV dysfunction and RHF; similarly, longer CPB time required for LVAD implantation was linked to worsening RHF in our study (1). Our finding of decreased perioperative hematocrit in more severe RHF is reflective of a prior study that found lower nadir hematocrit to be significantly associated with increased mortality in patients undergoing CPB (18). It also aligns with another study in which the need for blood transfusions was related to RHF severity (6). The decreased survival rates with increased RHF severity has also been previously documented (6,8,10,11).
Patients who develop RHF in the postoperative setting are at risk for developing multi-organ dysfunction, especially of the respiratory and renal systems. Our study demonstrated similar results. These conditions, along with postoperative mortality, are associated with the severity of RHF, further stressing the importance of volume, mechanical device, and natural cardiac optimization to limit RHF occurrence and progression. Awareness of RHF development and its consequences has increased over the years and novel strategies have been implemented in attempts to prevent its occurrence and limit its progression. Close echocardiographic ventricular monitoring with careful LVAD flow dynamic adjustments, non-pulsatile LVAD designs, and intra-operative RVAD placement prior to RHF development are a few innovations proven to reduce RHF based on clinical experience and research (1,3). Others include methods to decrease pulmonary hypertension, such as the use of nitric oxide gas and PDE-5 inhibitors perioperatively, and improve RV support, such as using the percutaneous RV Impella device (1,19,20).
RHF following LVAD implantation is an unfortunate complication with significantly negative implications for surgical outcomes. This increased risk of postoperative complications, including death, causes an urgent need for improved RHF risk stratification for LVAD candidates. Furthermore, the sensitivity to hemodynamic alterations exhibited in these patients cannot be overemphasized. A delicate balance of volume status, natural heart function, and mechanical function must be maintained, which is especially crucial immediately following the operation. Specialized patient care will be better directed as more is learned regarding the characteristics of patients who develop RHF. For example, our findings indicate that LVAD patients with higher BMI and pre-existing renal dysfunction could be considered at increased risk and may require additional monitoring in both the pre and post-operative settings. Our work also indicates that CPB time and volume shifts intraoperatively should be kept at a minimum, when clinically possible.
Limitations
These results came from a retrospective single-center observational experience and may not be generalizable. As the data were not initially recorded for the purpose of this study, potential variables of interest, such as transfusions or echocardiographic and hemodynamic parameters, were not available. Further, the full profiles of inotropes and vasopressors were not available for analysis; however, both inotropes and vasopressors have previously been identified as useful predictors (8,21). However, from the INTERMACS profiles, we know that pre-operative pressor-dependence was associated with the severity of RHF development, with approximately 11% of those without RHF having dependence, compared with approximately 63% of those with acute-severe RHF. While we found CPB time to be predictive of RHF development, it is possible that if severe hypotension develops while weaning from CPB, it may lead to somewhat longer CPB times until the hypotension is sufficiently controlled with vasopressors and/or other forms of support, such as RVAD placement (6).
Conclusions
This study reinforced RHF as a substantial risk following LVAD implantation, as well as being a predictor of subsequent poor clinical course. Further, we found that the risk for 1-year mortality increased significantly as the severity of RHF worsened. CPB duration and nadir hematocrit were jointly identified as risk factors for RHF and warrant further study. This work may help clinicians strategize preoperative preparation, intraoperative actions, and postoperative management in an effort to reduce RHF development, which may also improve other surgical outcomes.
Acknowledgements
This work was funded in part by the Baylor Health Care System Foundation.
Footnote
Conflicts of Interest: Dr. Joseph and Dr. Hall have received speaking honoraria from St. Jude Medical. The other authors have no conflicts of interest to declare.
Ethical Statement: The protocol for data collection was approved by the Institutional Review Board of Baylor University Medical Center Dallas (IRB File #011-274), and informed consent was waived.
References
- Meineri M, Van Rensburg AE, Vegas A. Right ventricular failure after LVAD implantation: prevention and treatment. Best Pract Res Clin Anaesthesiol 2012;26:217-29. [Crossref] [PubMed]
- Fida N, Loebe M, Estep JD, et al. Predictors and management of right heart failure after left ventricular assist device implantation. Methodist Debakey Cardiovasc J 2015;11:18-23. [Crossref] [PubMed]
- Dandel M, Krabatsch T, Falk V. Left ventricular vs. biventricular mechanical support: Decision making and strategies for avoidance of right heart failure after left ventricular assist device implantation. Int J Cardiol 2015;198:241-50. [Crossref] [PubMed]
- Lampert BC, Teuteberg JJ. Right ventricular failure after left ventricular assist devices. J Heart Lung Transplant 2015;34:1123-30. [Crossref] [PubMed]
- Chung BB, Sayer G, Uriel N. Mechanical Circulatory Support Devices: Methods to Optimize Hemodynamics During Use. Expert Rev Med Devices 2017;14:343-53. [Crossref] [PubMed]
- Kormos RL, Teuteberg JJ, Pagani FD, et al. Right ventricular failure in patients with the HeartMate II continuous-flow left ventricular assist device: incidence, risk factors, and effect on outcomes. J Thorac Cardiovasc Surg 2010;139:1316-24. [Crossref] [PubMed]
- Baumwol J, Macdonald PS, Keogh AM, et al. Right heart failure and "failure to thrive" after left ventricular assist device: clinical predictors and outcomes. J Heart Lung Transplant 2011;30:888-95. [PubMed]
- Dang NC, Topkara VK, Mercando M, et al. Right heart failure after left ventricular assist device implantation in patients with chronic congestive heart failure. J Heart Lung Transplant 2006;25:1-6. [Crossref] [PubMed]
- Santambrogio L, Bianchi T, Fuardo M, et al. Right ventricular failure after left ventricular assist device insertion: preoperative risk factors. Interact Cardiovasc Thorac Surg 2006;5:379-82. [Crossref] [PubMed]
- Matthews JC, Koelling TM, Pagani FD, et al. The right ventricular failure risk score a pre-operative tool for assessing the risk of right ventricular failure in left ventricular assist device candidates. J Am Coll Cardiol 2008;51:2163-72. [Crossref] [PubMed]
- Drakos SG, Janicki L, Horne BD, et al. Risk factors predictive of right ventricular failure after left ventricular assist device implantation. Am J Cardiol 2010;105:1030-5. [Crossref] [PubMed]
- Fitzpatrick JR 3rd, Frederick JR, Hsu VM, et al. Risk score derived from pre-operative data analysis predicts the need for biventricular mechanical circulatory support. J Heart Lung Transplant 2008;27:1286-92. [Crossref] [PubMed]
- Kalogeropoulos AP, Kelkar A, Weinberger JF, et al. Validation of clinical scores for right ventricular failure prediction after implantation of continuous-flow left ventricular assist devices. J Heart Lung Transplant 2015;34:1595-603. [Crossref] [PubMed]
- Interagency Registry for Mechanically Assisted Circulatory Support - Appendix A: Adverse Event Definitions: Adult and Pediatric patients, 2013.
- Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc 1999;94:496-509. [Crossref]
- , .Kidney Disease Improving Global Outcomes Clinical Practice Guideline for Acute Kidney Injury. 2012.
- Ochiai Y, McCarthy PM, Smedira NG, et al. Predictors of severe right ventricular failure after implantable left ventricular assist device insertion: analysis of 245 patients. Circulation 2002;106:I198-202. [PubMed]
- Loor G, Li L, Sabik JF 3rd, et al. Nadir hematocrit during cardiopulmonary bypass: end-organ dysfunction and mortality. J Thorac Cardiovasc Surg 2012;144:654-662.e4. [Crossref] [PubMed]
- MacGowan GA, Schueler S. Right heart failure after left ventricular assist device implantation: early and late. Curr Opin Cardiol 2012;27:296-300. [Crossref] [PubMed]
- Anderson MB, Goldstein J, Milano C, et al. Benefits of a novel percutaneous ventricular assist device for right heart failure: The prospective RECOVER RIGHT study of the Impella RP device. J Heart Lung Transplant 2015;34:1549-60. [Crossref] [PubMed]
- Soliman OII, Akin S, Muslem R, et al. Derivation and Validation of a Novel Right-Sided Heart Failure Model After Implantation of Continuous Flow Left Ventricular Assist Devices: The EUROMACS (European Registry for Patients with Mechanical Circulatory Support) Right-Sided Heart Failure Risk Score. Circulation 2018;137:891-906. [Crossref] [PubMed]