Bronchoscopic treatment of emphysema: an update
Chronic obstructive pulmonary disease (COPD) affects approximately 1.8% of the population (1,2); it is a leading cause of disability and death. Maximal medical treatment, although effective at the early stages of the disease, becomes limited when extensive alveolar destruction is the main cause of respiratory failure. For this reason there has been an increasing urge for surgical and nonsurgical treatment. Various surgical procedures have been proposed in the past: chostochondrectomy, thoracoplasty, phrenicectomy, pneumoperitoneum, pleural abrasion, denervation of the lung, glomectomy and many others (3,4); although they showed positive immediate results, an objective and prolonged benefit was rarely obtained; thus, they were progressively abandoned. Bullectomy is the only one that resisted up to now (5). Lung transplantation is certainly a viable option for a selected group of patients (6); however, the reduced number of available donors and the recent introduction of the lung allocation score (LAS) for organ allocation (7) significantly reduces the chances for COPD patients to reach the time of transplant. For this reason other options have been investigated. In 1993, lung volume reduction surgery (LVRS) revitalized (8,9). The pathophysiological principles of this operation are quite easy to understand: the removal of the worse parts of the emphysematous lung helps to remodel the diaphragmatic and chest wall mechanics. We are now aware that LVRS allows a reliable improvement in selected patients but the initial experience showed a significant in-hospital mortality of 19% (10). For this reason the NETT trial was designed (11,12); however, due to a number of problems it was able to answer only partially the questions raised by the initial experience. The subsequent review of these data helped to revitalize this procedure (13); it is now clear that patients with a more advanced functional deterioration (low DLCO), homogeneous emphysema, and a number of absolute and relative contraindications show less impressive results and a higher mortality.
Endoscopic alternatives to LVRS have recently gained acceptance and some of them are already extensively employed. They promote lung deflation searching the same outcome as LVRS: they improve respiratory mechanics and ameliorate the distressing symptom of chronic dyspnea by decreasing the physiological dead space.
Airway bypass
This procedure is based on collateral ventilation (CV) as was originally defined in 1930 by Van Allen (14): the ability of air to flow inside the lung through non-anatomical pathways. This phenomenon was further demonstrated by Hogg in 1969 (15) and Terry in 1978 (16). Three levels of CV are present in normal human lungs: the intra-alveolar pores of Khon, the accessory bronchiolar-alveolar connections of Lambert and the interbronchial channels of Martin (17,18). In emphysematous lungs the destruction of alveolar septa creates a preferential route for collateral air flow, as a result of inflammatory or sheer force damage between airways and lung parenchyma (18,19). Macklem suggested that the lower resistance through collateral channels might show startling therapeutic implications (20): he suggested that the creation of extra-anatomic communications between the lung surface and the skin (through the thoracic wall) may contribute to deflate the lungs bypassing the obstructed small airways, allowing trapped air to exit from the hyperinflated lungs. This was certainly a great idea but it could create problems in a clinical scenario.
The “airway bypass” was subsequently revitalized by Joel Cooper (21). He suggested that the creation of communications between the parenchyma and bronchi would allow lung deflation and improve expiratory flow and respiratory mechanics. The new pathways would bypass during expiration the obstructed small airways, particularly in patients with homogeneous emphysema.
After a number of laboratory and ex vivo experiments confirming the feasibility of the procedure, a safety study was performed in patients undergoing lobectomy and lung transplantation (22). A miniaturized endobronchial Doppler probe was used to scan the extrabronchial vessels; a radiofrequency probe was initially used to create the extranatomical transbronchial passages. However, the radial spread of heath from the probe injured the adjacent tissue; also, the risk of penetrating too deeply increased the risk of potential pneumothorax and hemorrhage. This problem was solved with a modification of the technique (23). After mapping the extrabronchial vessels, instead of the radiofrequency probe a 22-gauge transbronchial needle was used to open a passage towards the parenchyma; aspiration through the needle confirmed the absence of vessels outside the bronchial wall in the scanned area. The fenestration was subsequently dilated with an angioplasty catheter with an expandable balloon. A balloon expandable stainless steel stent covered with silicone was subsequently deployed into the passage. In order to avoid or delay the growing of granulations, mitomycin C was delivered over the stent. In dogs this procedure allowed to keep the fenestrations open for up to 20 weeks. The disadvantages of separate needle and balloon devices were overcome by a combined probe including both of them. To improve patency a Paclitaxel eluting stent was developed; this technique strongly contributed to keep the fenestrations open for a longer period of time (24).
The largest trial, performed on 208 patients, showed functional improvement at one month; however, this improvement was not sustained at 3, 6 and 12 months (25,26). Most of the stents were lost due cough and expectoration; also granulation and occlusion were significant problems (27). A recent meta-analysis confirmed that among the available endoscopic procedures the airway bypass had the least impressive results to date (28).
Endoscopic one-way valves
The background for this procedure is based on the assumption that blocking the airway supplying the most overinflated areas of emphysematous lungs could cause atelectasis, mimicking the effect of LVRS on respiratory mechanics. This was originally demonstrated in 2001 by Ingenito et al. (29) in his experimental work. There are two devices employed in the clinical practice: the umbrella-like “intrabronchial valve “(IBV) and the mouth-fish appearing “endobronchial valve” (EBV). Both valves are unidirectional and should be deployed in the segmental bronchi to allow deflation of the target area.
IBV is made of a nitinol framework with five anchors seated distally that engage the airway and provide stability. The proximal part of the valve is made up of six struts expanding radially and covered by an umbrella of synthetic polymer that adheres to the airway wall and “seals” with minimal pressure on the mucosa. There is a proximal central rod facilitating grasping for repositioning or removal. The valve limits airflow distally but allows mucociliary clearance and decompression.
This device has been evaluated in a North American multicenter trial (30). In a randomized trial, 277 patients with severe COPD were enrolled at 36 centers. The primary effectiveness measures were an improvement in the St. George’s Respiratory Questionnaire (SGRQ) and lobar volume modifications. The assessment of serious adverse events was the primary safety measure. Five per cent of the patients in the treatment group (6/121) were responders at 6 months; this percentage was significantly higher than the control group [1/134 (0.7%)]. There were also significative differences in terms of lobar volume changes: −224 mL in the valve group compared with −17 mL for the control group (sham procedure). However, the proportion of responders in terms of SGRQ was not higher in the treatment group. There were more serious adverse events in the treatment group [20 (14.1%) vs. 5 (3.7%)]; however, most of them were neither procedure nor device related. There is growing evidence that this type of valves work less effectively than the other one (31).
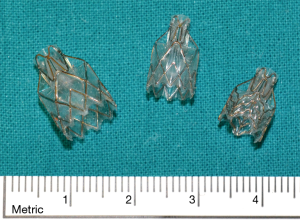
Also the EBV is a one-way structure preventing air entering the target area but allowing air and mucous to exit (Figure 1). The valve is included in a stent-like self-expanding retainer that secures it in place during respiration and coughing. The retainer is covered with silicone to “seal” the valve to the bronchus. The device is provided in two sizes: the 4.0 for bronchial lumens ranging between 4 and 7 mm in diameter, and the 5.5, for diameters ranging between 5.5 and 8.5 mm. A flexible delivery catheter is used to deploy this device into the bronchi. A bronchial diameter measurement gauge is attached to the proximal end of the distal housing to help choosing the valve of the appropriate size. On the delivery catheter for the Zephyr EBV 4.0 the larger gauge spans a 7-mm diameter and the smaller gage spans a 4-mm diameter, indicating the maximum and minimum treatable bronchial diameters respectively for this size of device. The EBV is compressed into the retractable distal housing using a dedicated loading system. The loaded catheter is advanced to the target bronchus and the valve is deployed. The delivery catheter can be inserted through a 2.8-mm flexible bronchoscope working channel. The EBV can be placed under local or general anaesthesia and are radiologically well visible.
After a series of preliminary experimental and clinical studies demonstrating safety and efficacy (32-37), the EBV were assessed with an international, prospective, multicentre, randomized study: the VENT (Endobronchial Valve for Emphysema Palliation Trial) study (38). This trial was performed at 54 centers in the USA and Europe and included 492 patients randomized after a period of 6–8 weeks of rehabilitation into a valve treatment group and the control group receiving standard medical treatment. The two cohort studies (Europe group—23 centers with 171 patients; USA group—31 centers with 321 patients) were published separately (39,40). In the USA study there where statistically significant functional changes in the valve group compared with the controls; however, the relevance of these changes was limited: FEV1 +4.3%, 6-minute walk test (6MWT) +2.5% and a small change in terms of SGRQ. Notwithstanding these controversial results, if the analysis was limited to patients with higher heterogeneity at computed tomography (CT), there was clearly a greater improvement in the valve group (FEV1 +10.7% and 6MWT +12.4%). A better response was also predicted by the presence of complete interlobar fissures at HRCT with consequent lower or absent interlobar CV; this would allow complete atelectasis of the occluded lobe. Similar results were observed in the European group. The safety of valve treatment was demonstrated in both reports with a very low incidence of complications: pneumonia, hemoptysis, exacerbations of COPD and pneumothorax.
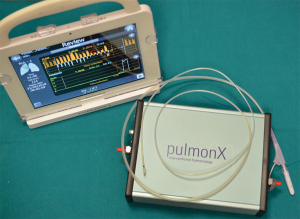
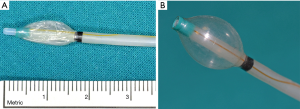
These preliminary results were subsequently confirmed by other multicenter studies (41,42). These studies showed that interlobar CV assessment is crucial for patient selection. Interlobar fissures can reliably be evaluated at High Resolution Computed Tomography (HRCT). More recently, a dedicated system has been assessed for this purpose: the Chartis System (Pulmonx Inc., Redwood, California, USA) (Figure 2) (43). This is an endobronchial probe with a balloon at his tip (Figure 3A,B); the inflation of the balloon blocks air entering into the target lobar bronchus; a console detects flow and pressure of air coming distally to the occluded bronchus. In the absence of recorded air flow, interlobar CV is assumed to be limited or absent. The measured resistance well correlates with post valve implantation atelectasis (44). Patients without interlobar CV are considered optimal candidates for EBV implantation. The accuracy of this system was assessed and confirmed in several studies and it is about 75% (34,45,46). An anatomical study assessing the correlations between the Chartis system and HRCT in patients undergoing pulmonary lobectomy for lung cancer (47) confirmed the previous conclusions. This study supported the idea that the cumulative information provided by these two techniques allows a reliable assessment of the anatomical interlobar fissures status and it improves patient selection for EBV treatment. Marshall (48) recently proposed a new technique to assess CV with a novel magnetic resonance imaging (MRI) method during a single breath-hold. However, this method requires further validation and correlation with lung heterogeneity, with the previously described methods and, finally, with outcome after valve treatment (49).
Endoscopic one way valve deployment, when associated with functional improvement, beneficially impacts right ventricular functional parameters (50). Speckle tracking-based right ventricular apical longitudinal strain analysis allows early determination of right ventricular contractile gain.
Medium and long-term assessment of patients undergoing EBV implantation is now available. In a group of 40 patients with a median follow up of 32 months (33 patients evaluated at 1 year, 18 at 3 years and 9 at 5 years) long-term sustained improvements were achieved (51); particularly, at 3 and 5 years it was still possible to observe a significant improvement in terms of supplemental O2 requirement, FEV1, FVC, RV, 6MWT and Medical Research Council (MRC) score. However, as for LVRS, the functional improvement obtained by EBV progressively declines to pre-treatment levels. For this reason, a second contralateral treatment has been successfully attempted after unilateral LVRS (52,53). This strategy was performed also after valve implantation with encouraging results (54).
After the VENT trial, other RCTs have been published: the STELVIO (55,56); the BELIEVER-HIFI (57), the TRANSFORM (58) and the IMPACT (59).
The STELVIO trial recruited 68 patients with severe COPD without CV; they were randomized between the EBV group (34 patients) and a control group receiving standard medical care. All of them had a complete or nearly complete fissure between the target lobe and the adjacent lobe at HRCT. Primary outcomes were 6 months modifications in term of FEV1, FVC and 6MWT. The increase in FEV1 was greater in the valve group by 140 mL; the FVC increase was greater by 347 mL; the increase in the 6MWT was greater by 74 meters; all the comparisons were statistically significant (P<0.01). Twenty-three adverse events were reported in the valve group (including one death) and 5 in the control group (P<0.001). Treatment related adverse events included pneumothorax (18%) and events requiring replacement or removal of the valve (12% and 15% respectively). Patients receiving standard medical treatment were eventually crossed over and treated with EBV after 6 months of follow-up and subsequently followed for another six months (56). After one year of follow up significant improvements (P<0.001) was still observed for FEV1 (+17%), RV (−687 mL), 6MWT (+61 meters) and SGRQ (−11 points). Two patients died (1 after 58 days due to progressive respiratory failure and 1 after 338 days due to myocardial infarction). Seventeen percent of the patients underwent valve replacement and 22% had permanent removal of the device. No pneumothorax occurred between 6 and 12 months after treatment. The authors concluded that “EBV treatment resulted in clinically relevant benefits at one year. Maintenance bronchoscopies to achieve this are needed”.
The BeLieVeR-HIFI (57) and the Transform study (58), performed in patients with heterogeneous emphysema confirmed the previously reported results.
The IMPACT study (59) published by Valipour and colleagues in 2016 enrolled 93 subjects with homogeneous emphysema and absence of CV assessed by the Chartis system. They were randomized 1:1 to EBV treatment or standard medical care. At 3 months there was a significative functional improvement in the EBV group as assessed by FEV1 (P=0.0002). Also the other variables showed significative improvement. In 11 patients a pneumothorax occurred. Five subjects required removal or replacement of the valves. The authors concluded that “EBV in patients with homogeneous emphysema without CV results in clinically meaningful benefits of improved lung function, exercise tolerance and quality of life”.
The efficacy of endobronchial allowed to enlarge the indications: nowadays valves are placed both before lung transplantation, as a bridge to transplant (60), and after single lung transplantation, to reduce hyperinflation of the native lung (61). EBV have been used also in patients with bullous emphysema (62) or to treat persistent air leaks (63,64).
Bronchial thermal vapor ablation (BTVA) therapy
This technique uses steam (Uptake Medical Corporation, Seattle, Washington, USA) to produce thermal injury of a target lung area. It is intended to reduce lung volumes, usually in heterogeneous emphysema, regardless of the presence of CV. The system consists of a generator and a dedicated catheter able to deliver water vapor directly into the lung at a precise amount of energy. This produces thermal damage, blood flow reduction and inflammation; it results in permanent scarring and fibrosis (32,65). Patients with higher inflammatory responses can achieve better clinical results. In experimental studies the amount of histologic fibrosis clearly correlates with atelectasis (66); the amount of volume reduction is dose-dependent (67). A multicenter trial was performed on 44 patients (68); this study included only those with heterogeneous upper lobe emphysema and it demonstrated that after six months there was a significant improvement in FEV1, FVC, RV, quality of life, dyspnea index and 6MWT. With this technique, the presence of interlobar fissures has no impact on results in terms of volume reduction of the lobe (69). Major complications were COPD exacerbations, pneumonia, respiratory tract infections and hemoptysis. The major drawback of this technique is its irreversibility.
The 6 and 12 months results of a multicenter RCT (STEP UP trial) were recently published (70,71). In this trial, the vapor technique was used in a step-up approach. This strategy is particularly useful in case of segmental heterogeneity, allowing treatment of the most emphysematous subcomponents of the lobe. The primary endpoints were changes in FEV1 and SGRQ; 70 patients were randomized. After 6 months the mean improvement in these two variables were was 14.7% and 9.7 points; both were statistically significative. COPD exacerbations were the most common serious adverse event (11 patients out of 45 in the treatment group and 1 out of 24 among the controls). The 12-month data have subsequently been published (71), demonstrating durable improvement. The between-group difference was a statistically significative 12.8% for FEV1 and −12.1 units for the SGRQ. RV showed an average reduction of 237 mL when compared to the controls. Most of the adverse events occurred within 90 days after treatment and all of them resolved with standard medical care with the exception of one; after 90 days, the incidence of adverse events was equal in the two arms (16% and 17%).
Coils
Coils (RePneu, PneumRx Inc., Mountain View, California; USA) are designed to act as spring elements able to retract the lung parenchyma towards the hilum by torqueing the bronchi. This technique, in contrast to EBV, is a nonocclusive procedure; it should allow to reduce lung volume, restore lung parenchyma tension and radial suspension of the peripheral airways (32).
The device consists of two components: the coil and the delivery system; the latter has a loading cartridge, a delivery catheter and forceps. The coils are made of a shape-memory nitilol wire with a length ranging between 100 and 150 mm. The deployment procedure is performed under fluoroscopy. The guide wire is initially advanced into the airway; the delivery catheter is then passed over the wire and the airway length is measured using radio opaque markers in order to choose the appropriate coil. The guide wire is removed and a preloaded straightened coil is advanced in place over the catheter. The catheter is removed leaving the coil in place within the target bronchus; at this point the coil returns to its predetermined shape torqueing the airway and retracting the parenchyma. The coil is then released from the biopsy forceps. Ten to twelve coils are usually released in the upper lobe and 10 to 14 in the lower lobes. Both sides should be treated with two separate bronchoscopic procedures separated approximately by 1–2 months (72). The coils can be deployed under deep sedation or general anesthesia.
The coils should work retracting and compressing the most damaged areas of the lung; this would reduce airflow in these areas and shift it to the healthier untreated parts of the lung; it also contributes to reduce hyperinflation and improves respiratory mechanics resetting the diaphragm and other inspiratory muscles (73). Coils should also ameliorate the elastic recoil of the lung, which could prevent airway collapse and air trapping, and improve expiratory flow. The outcome of coil treatment does not depend on the absence of CV since it consists in a mechanical retraction of the parenchyma (74). This treatment is not fully reversible, although removal of single coils has been reported (47). The use of coils require is not indicated if the lung is too destroyed or if there are large bullae since they require a minimal amount of “viable” parenchyma to optimize performance.
Several studies have investigated feasibility, safety and efficacy of coil treatment. Six studies (74-79) have been involved in an analysis of this technique (73). One hundred-sixty-eight patients were enrolled in these six studies; 122 of them were treated bilaterally. Five to fifteen coils per procedure were delivered; the median time per procedure was approximately 40 minutes (range, 40–135 minutes). These studies showed that the procedure was feasible and well tolerated. No perioperative adverse events were recorded and no coils removal was required, confirming safety. During the first 30 days after coil deployment 11 patients had a pneumothorax (6.5%); COPD exacerbations were also reported, as well as mild, self-limiting hemoptysis and transient chest discomfort (73). During the following six months of follow-up no adverse events were reported. The functional efficacy of the first reported study was certainly less impressive (74). This was probably related to the use of a reduced number of coils and the use of first generation devices. For this reason, the functional analysis of that study (73) included only the following 5 studies, that were methodologically more uniform. Those studies reported a six-month increase for FEV1 of 13%; RV decreased approximately 0.4 liters. At the same time point there was an average increase in 6MWT of 30–84 meters. Quality of life, measured by the SGRQ, decreased of 6–15 points. Dyspnea severity measured by mMRC score showed a decrease of 0.6 points.
A large randomized clinical trial (REVOLENS trial) (80) was recently published by Deslée and colleagues. This study was designed with a 6-month follow up and it showed that at this time point coils deployment allowed improved exercise capacity with high short-term costs. One hundred patients were randomized into two groups: those receiving standard medical treatment only (50 patients) and those receiving bilateral coil treatment (10 coils per lobe) in addition to standard medical therapy. The primary end point was the improvement of at least 54 meters in the 6MWT; the secondary outcomes included 6 and 12 months modifications in the 6MWT, lung function, SGRQ (range 0–100; 0 being the best and 100 the worse; minimal clinical important difference, ≥4), morbidity, mortality, cost and cost-effectiveness. At 6 months, the primary end point was observed in 36% of the patients in the coil group and 18% in the usual care group (P=0.03). The mean between-group difference (coil and standard group) at 6 and 12 months for FEV1 was respectively +0.09 L (P=0.01) and +0.08 L (P=0.002), for 6MWT was +21 (P=0.06) and +21 meters respectively (P=0.12), for the SGRQ it was −13.4 and −10.6 points (P=0.01 for both). Four deaths occurred within 12 months in the coil group and 3 in the other group. The mean one year per-patient cost difference between was $47,908 (P<0.001); the incremental cost-effectiveness ratio was $782,598 per additional quality-adjusted life-year. The authors conclude that in that study “treatment with coils resulted in improved exercise capacity at 6 months with higher short term costs”. Long term follow up (up to 3 years) (81) demonstrates that clinical benefit declines over time; however, at 3 years post-treatment, 50% of the patients maintain improvement in terms of 6MWT, SGRQ and mMRC.
In 2016 Sciurba and colleagues (82) published a RCT on 315 patients with emphysema enrolled in 21 North American and 5 European centers (the RENEW randomized clinical trial). Patients were randomized into two groups: standard medical treatment including rehabilitation (157 patients) and standard treatment plus two sequential bilateral coil procedures 4 months apart (10 to 14 coils deployed in a single lobe) (158 patients). The primary outcome was the difference in change in 6MWT at 12 months [minimal clinically important difference (MCID) =25 meters]. The secondary end points included the difference in terms of 6MWT responder rate, the absolute change in SGRQ (MCID =4) and change in FEV1 (MCID =10%). Safety was assessed by comparing the proportion of patients with at least 1 out of predefined major complications. The median change in 6MWT at 12 months was 10.3 m in the coil group vs. 7.6 m in the other group (P=0.02). Improvement of at least 25 meters occurred in 40% of the patients in the coil group and 26.9% in the standard care group (P=0.1). The difference in FEV1change was 7.0% (P=0.001), and the SGRQ score improved −8.9 points (P=0.001), each in favor of the coil group. Major complications occurred in 34.8% of the patients in the coil group and 19.1% in the standard therapy group (P=0.002). Serious adverse events included pneumonia (20% and 4.5% in the coil and standard group respectively) and pneumothorax (9.7% and 0.6%) occurred more frequently in the coil group. The authors concluded that “the use of endobronchial coils compared with standard therapy resulted in an improvement in median exercise tolerance that was modest and of uncertain clinical importance, with higher likelihood of major complications”.
These studies demonstrated that this treatment can be considered an option for patients with severe emphysema with or without CV and both homogeneous and heterogeneous emphysema. Further randomized trials will increase the knowledge of the treatment’s efficacy.
Biological lung volume reduction (sealant)
The instillation of sealants within the airway was originally named “biological lung volume reduction” (29,83). This procedure was designed to produce irreversible lung volume reduction by closing the airway and blocking CV pathways; this goal was accomplished by injecting sealants into the lung parenchyma down to the alveoli to induce inflammation and subsequent fibrosis (84). After the initial attempts with biological substances (85), a synthetic polymeric foam was used (Aeris Therapeutics, Woburn Mass, USA) (40). A study with unilateral occlusion published by Herth and colleagues (40) showed a significant improvement of FEV1, FVC and SGRQ after 24 weeks in 21 patients.
Kramer and colleagues (86) performed a bilateral treatment including both patients with heterogeneous and homogeneous emphysema. They occluded two subsegments in each upper lobe. CT analysis showed a significative decrease in upper lobe volume along with improvements in FEV1 and SGRQ. However, one case of treatment-related death was observed. This procedure acts at the alveolar level and thus is not influenced by CV. However, it is clearly irreversible. The doses of the agent and the instillation methods are still under evaluation.
Based on the promising results of open-label pilot studies, the AeriSeal System for Hyperinflation Reduction in Emphysema (ASPIRE) study was initiated (87). This was a multicenter prospective, randomized controlled trial (RCT) comparing optimal medical therapy alone to ELS (emphysematous lung sealant system) plus optimal medical therapy in patients with upper lobe predominant disease. However, that study was prematurely stopped for business-related reasons, when only 95 patients were enrolled (300 were planned). These results were subsequently published since they provided sufficient data for a 3- and 6-month analysis. In the treatment group, 3-month lung function, dyspnea and quality of life significantly improved when compared to controls (the FEV1 improved by 11.4%); improvements persisted at 6 months with more than 50% of the patients experiencing clinically relevant amelioration of lung function; 44% of patients experienced adverse events requiring hospitalization; two deaths were observed in the treatment group. Curiously, the percentage of responders was higher among those with adverse events. Thus, this technique should be used only in patients included in clinical trials in well selected centers.
Targeted lung denervation
Surgical lung denervation has been proposed several decades ago without objective stable functional improvement. In 2015 Slebos and colleagues (88) published a study assessing a minimally invasive endoscopic system able to provoke targeted lung denervation. This system allows disruption of the parasympathetic bronchial innervation by a RF-energy releasing system; this allows to reduce acetylcholine in the airway with a permanent anti-cholinergic effect. They treated 22 patients demonstrating the feasibility of the procedure. The study showed a better outcome, although not uniformly statistically significant, when higher energy was administered. One-year changes comparing the 20 W dose with the 15 W dose of energy showed improvements in FEV1 (+11.6% vs. +0.02), submaximal cycle endurance (+6.8 vs. +2.6 min) and SGRQ (−11.1 vs. −0.9 points). Fifty-nine percent of the patients had COPD exacerbations during the first year. The first RCT evaluating this technology is underway (ClinicalTrials.gov, NCT02058459).
Liquid nitrogen metered cryospray
This technique allows delivering endoscopically liquid nitrogen within the airway to elicit cryoablation at a depth of 0.1 to 0.5 mm in patients with chronic bronchitis. This technique should initially induce crio-necrosis of the hyperplastic goblet cells and excess submucosal glands and subsequently favor regenerative airway healing and rejuvenation of the normal epithelium (89). The first human trials testing this technology are currently underway (NCT02106143, NCT02483052, NCT02483637).
To close this review, we believe that a recent meta-analysis is worth being reported (90). The authors performed this analysis on RCTs on bronchoscopic lung volume reduction searched on PubMed, Embase and the Cochrane library and reference list of related articles. A total of 1,802 records were retrieved: 385 were duplicates and thus excluded, 1,369 were excluded based on abstract and the 48 were assessed for eligibility. Only techniques with more than 2 RCTs were included in the analysis; thus, 1 RCT for lung sealants, 1 for bronchial vapour ablation and 1 for airway bypass were excluded. At the end of the selection process 6 studies on the EBVs (Zephyr) (39,40,55,57,59,91), 2 on intrabronchial valves (IBV, Spiration) (30,42) and 3 with endobronchial coils (77,80,82) were included. Compared with conventional therapy, endobronchial coils showed a better response in MCID for FEV1 (P<0.0001), 6MWT (P=0.01) and SGRQ (P<0.00001). Also EBV showed significative improvements in FEV1 (P=0.002), 6MWT (P=0.01) and SGRQ (P=0.0002). Notwithstanding these statistically significant improvements, both techniques were accompanied by serious adverse events. In the coils group there was no difference between homogeneous and heterogeneous emphysema. The IBV group did not show superior results when compared with the conventional group. The authors concluded that coils or EBV could significantly improve pulmonary function, exercise capacity and quality of life when compared with standard medical treatment. Coils could be deployed also in patients with homogeneous emphysema, although further RCTs are required.
Several methods are currently available for endoscopic treatment of emphysema. All of them are relatively safe. The efficacy of many of these techniques is related to the absence of CV and to the presence of heterogeneous disease. However, few procedures can be performed also in patients with CV. For all of them further studies are required to produce data able to improve selection of patients and reliably predict a favorable outcome. A rigorous follow-up is mandatory.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Global initiative for Chronic Obstructive Pulmonary Disease (GOLD initiative). Available online: http://www.goldcopd.org/
- Viegi G, Pistelli F, Sherrill D, et al. Definition, epidemiology and natural history of COPD. Eur Respir J 2007;30:993-1013. [Crossref] [PubMed]
- Cooper JD. The history of surgical procedures for emphysema. Ann Thorac Surg 1997;63:312-9. [Crossref] [PubMed]
- Deslauriers J. History of surgery for emphysema. Semin Thorac Cardiovasc Surg 1996;8:43-51. [PubMed]
- De Giacomo T, Rendina EA, Venuta F, et al. Bullectomy is comparable to lung volume reduction in patients with emphysema. Eur J Cardiothorac Surg 2002;22:357-62. [Crossref] [PubMed]
- Venuta F, Van Raemdonck D. History of lung transplantation. J Thorac Dis 2017;9:5458-71. [Crossref] [PubMed]
- Gottlieb J. Lung allocation. J Thorac Dis 2017;9:2670-4. [Crossref] [PubMed]
- Cooper JD, Trulock EP, Triantafillou AN, et al. Bilateral pneumonectomy (volume reduction) for chronic obstructive pulmonary disease. J Thorac Cardiovasc Surg 1995;109:106-16; discussion 116-9. [Crossref] [PubMed]
- Brantigan OC, Muller E. Surgical treatment of pulmonary emphysema. Am Surg 1957;23:789-804. [PubMed]
- Lefrak SS, Yusen RD, Trulock EP, et al. Recent advances in surgery for emphysema. Annu Rev Med 1997;48:387-98. [Crossref] [PubMed]
- Fishman A, Martinez F, Naunheim K, et al. National emphysema treatment trial research group. A randomized trial comparing lung volume reduction surgery with medical therapy for emphysema. N Engl J Med 2003;348:2059-73. [Crossref] [PubMed]
- National Emphysema Treatment Trial Research Group, Fishman A, Fessler H, et al. Patients at high risk of death after lung volume reduction surgery. N Engl J Med 2001;345:1075-83. [Crossref] [PubMed]
- Sanchez PG, Kucharczuk JC, Su S, et al. National Emphysema Treatment Trial the redux: accentuating the positive. J Thorac Cardiovasc Surg 2010;140:564-72. [Crossref] [PubMed]
- Van Allen CM, Lindskog GE, Richter HT. Gaseous interchange between adjacent lung lobules. Yale J Biol Med 1930;2:297-300. [PubMed]
- Hogg JC, Macklem PT, Thurlbeck WM. The resistance of collateral channels in excised human lungs. J Clin Invest 1969;48:421-31. [Crossref] [PubMed]
- Terry PB, Traystman RJ, Newball HH, et al. Collateral ventilation in man. N Engl J Med 1978;298:10-5. [Crossref] [PubMed]
- Lambert MW. Accessory bronchoalveolar communications. J Pathol Bacteriol 1955;70:311-4. [Crossref] [PubMed]
- Mitzner W. Collateral ventilation. In: Crystal RG. editor. The lung: scientific foundations. New York, NY: Raven Press, 1991:1053-63.
- Delaunois L. Anatomy and physiology of collateral ventilation in man. Eur Respir J 1989;2:893-904. [PubMed]
- Macklem PT. Collateral ventilation. N Engl J Med 1978;298:49-50. [Crossref] [PubMed]
- Lausberg HF, Chino K, Patterson GA, et al. Bronchial fenestration improves expiratory flow in emphysematous human lungs. Ann Thorac Surg 2003;75:393-7; discussion 398. [Crossref] [PubMed]
- Rendina EA, De Giacomo T, Venuta F, et al. Feasibility and safety of the airway bypass procedure for patients with emphysema. J Thorac Cardiovasc Surg 2003;125:1294-9. [Crossref] [PubMed]
- Choong CK, Haddad FJ, Gee EJ, et al. Feasibility and safety of airway bypass stent placement and influence of topical mitomycin C on stent patency. J Thorac Cardiovasc Surg 2005;129:632-8. [Crossref] [PubMed]
- Choong CK, Phan L, Massetti P, et al. Patency of airway bypass stents is prolonged with use of drug eluting stents. American Association for Thoracic Surgery 85 Annual Meeting; April 10-13, 2005, San Francisco, CA; Paper F20, page 160.
- Cardoso PF, Snell GI, Hopkins P, et al. Clinical application of the airway by pass with paclitaxel-eluting stents: early results. J Thorac Cardiovasc Surg 2007;134:974-81. [Crossref] [PubMed]
- Shah PL, Slebos DJ, Cardoso PF, et al. Bronchoscopic lung volume reduction with Exhale airway stents for emphysema (EASE trial):randomized, sham-controlled, multicenter trial. Lancet 2011;378:997-1005. [Crossref] [PubMed]
- Neder JA, O’Donnell DE. Update on nonsurgical lung volume reduction procedures. Can Respir J 2016;2016:6462352. [Crossref] [PubMed]
- Iftikhar IH, McGuire FR, Musani AI. Efficacy of bronchoscopic lung volume reduction: a meta-analysis. Int J Chron Obstruct Pulmon Dis 2014;9:481-91. [Crossref] [PubMed]
- Ingenito EP, Reilly JJ, Mentzer SJ, et al. Bronchoscopic volume reduction: a safe and effective alternative to surgical therapy for emphysema. Am J Respir Crit Care Med 2001;164:295-301. [Crossref] [PubMed]
- Wood DE, Nader DA, Springmeyer S, et al. The IBV valve trial: a multicenter, randomized, double-blind trial of endobronchial therapy for severe emphysema. J Bronchology Interv Pulmonol 2014;21:288-97. [Crossref] [PubMed]
- Gompelmann D, Eberhardt R, Herth F. Endoscopic volume reduction in COPD-a critical review. Dtsch Arztebl Int 2014;111:827-33. [PubMed]
- Gasparini S, Zuccatosta L, Bonifazi M, et al. Bronchoscopic treatment of emphysema: state of the art. Respiration 2012;84:250-63. [Crossref] [PubMed]
- Snell GI, Halsworth L, Borrill ZL, et al. The potential for Bronchoscopic lung volume reduction using bronchial prosthesis: a pilot study. Chest 2003;124:1073-80. [Crossref] [PubMed]
- Toma TP, Hopkinson NS, Hiller J, et al. Bronchoscopic volume reduction with valve implants in patients with severe emphysema. Lancet 2003;361:931-3. [Crossref] [PubMed]
- Hopkinsons NS, Toma TP, Hansell DM, et al. Effect of Bronchoscopic lung volume reduction on dynamic hyperinflation and exercise in emphysema. Am J Crit Care Med 2005;171:423-4.
- Yim AP, Hwong TM, Lee TW, et al. Early results of endoscopic lung volume reduction for emphysema. J Thorac Cardiovasc Surg 2004;127:1564-73. [Crossref] [PubMed]
- Venuta F, De Giacomo T, Rendina EA, et al. Bronchoscopic lung volume reduction with one way valves in patients with emphysema. Ann Thorac Surg 2005;79:411-6; discussion 416-7. [Crossref] [PubMed]
- Strange C, Hearth FJ, Kovitz KL, et al. Design of the Endobronchial Valve for Emphysema Palliation Trial (VENT): a nonsurgical method of lung volume reduction. BMC Pulm Med 2007;7:10. [Crossref] [PubMed]
- Sciurba FC, Ernst A, Herth FJ, et al. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med 2010;363:1233-44. [Crossref] [PubMed]
- Herth FJ, Noppen M, Valipour A, et al. Efficacy predictors of lung volume reduction with Zephyr valves in an European cohort. Eur Respir J 2012;39:1334-42. [Crossref] [PubMed]
- Sterman DH, Metha AC, Wood DE, et al. A multicentre pilot study of a bronchial valve for the treatment of severe emphysema. Respiration 2010;79:222-33. [Crossref] [PubMed]
- Ninane V, Geltner C, Bezzi M, et al. Multicentre European study for the treatment of advanced emphysema with bronchial valves. Eur Respir J 2012;39:1319-25. [Crossref] [PubMed]
- Mantri S, Macaraeg C, Shetty S, et al. Measurement of collateral flow in the lung with a dedicated endobronchial catheter system. J Bronchology Interv Pulmonol 2009;16:141-4. [Crossref] [PubMed]
- Gompelmann D, Eberhardt R, Michaud G, et al. Predicting atelectasis by assessment of collateral ventilation prior to endobronchial lung volume reduction: a feasibility study. Respiration 2010;80:419-25. [Crossref] [PubMed]
- Herth FJ, Eberhardt R, Gompelmann D, et al. Radiological and clinical outcomes of using Chartis to plan endobronchial treatment. Eur Respir J 2013;41:302-8. [Crossref] [PubMed]
- Gompelmann D, Ralf E, Slebos DJ, et al. Comparison between Chartis pulmonary assessment system detection of collateral ventilation vs corelab CT fissure analysis in predicting atelectasis in emphysema patients treated with endobronchial valves. Abstract presented at ERS annual congress; 2012, Vienna, Austria.
- Diso D, Anile M, Carillo C, et al. Correlation between collateral ventilation and interlobar lung fissures. Respiration 2014;88:315-9. [Crossref] [PubMed]
- Marshall H, Deppe MH, Parra-Robles J, et al. Direct visualizzazioni of collateral ventilation in COPD with hyperpolarized gas MRI. Thorax 2012;67:613-7. [Crossref] [PubMed]
- Anile M, Diso D, Venuta F. Assessment of intraparenchymal lung collateral ventilation. Thorax 2012;67:1111-author reply 1111. [Crossref] [PubMed]
- Pizarro C, Schueler R, Hammerstingl C, et al. Impact of endoscopic lung volume reduction on right ventricular myocardial function. Plos One 2015;10:e0121377. [Crossref] [PubMed]
- Venuta F, Anile M, Diso D, et al. Long-term follow up after bronchoscopic lung volume reduction in patients with emphysema. Eur Respir J 2012;39:1084-9. [Crossref] [PubMed]
- Kostron A, Horn-Tutic M, Franzen D, et al. Repeated lung volume reduction surgery is successful in selected patients. Eur J Cardiothorac Surg 2015;48:710-5. [Crossref] [PubMed]
- Tacconi F, Pompeo E, Forcella D, et al. Lung volume reduction reoperations. Ann Thorac Surg 2008;85:1171-7. [Crossref] [PubMed]
- Fiorelli A, D’Andrilli A, Anile M, et al. Sequential bilateral bronchoscopic lung volume reduction with one-way valves for heterogeneous emphysema. Ann Thorac Surg 2016;102:287-94. [Crossref] [PubMed]
- Klooster K, ten Haken NHT, Hartman JE, et al. Endobronchial valves for emphysema without interlobar collateral ventilation. N Engl J Med 2015;373:2325-35. [Crossref] [PubMed]
- Klooster K, Hartman JE, Ten Hacken NH, et al. One-year follow up after endobronchial valve treatment in patients with emphysema without collateral ventilation treated in the STELVIO trial. Respiration 2017;93:112-21. [Crossref] [PubMed]
- Davey C, Zoumot A, Jordan S, et al. Bronchoscopic lung volume reduction with endobronchial valves for patients with heterogeneous emphysema and intact interlobar fissures (the BeLieVer-HIFI study): a randomised controlled trial. Lancet 2015;386:1066-73. [Crossref] [PubMed]
- Kemp SV, Slebos DJ, Kirk A, et al. A multicentre randomized controlled trial of Zephyr endobronchial valve treatment in heterogeneous emphysema (TRASFORM). Am J Respir Crit Care Med 2017;196:1535-43. [Crossref] [PubMed]
- Valipour A, Slebos DJ, Herth F, et al. Endobronchial valve therapy in patients with homogeneous emphysema. Am J Respir Crit Care Med 2016;194:1073-82. [Crossref] [PubMed]
- Venuta F, Diso D, Anile M, et al. Bronchoscopic lung volume reduction as a bridge to lung transplantation in patients with chronic obstructive pulmonary disease. Eur J Cardiothorac Surg 2011;39:364-7. [Crossref] [PubMed]
- Destors M, Aniwidyaningsih W, Jankowski A, et al. Endoscopic volume reduction before and after lung transplantation. Eur J Cardiothorac Surg 2012;42:897-8. [Crossref] [PubMed]
- Santini M, Fiorelli A, Vicidomini G, et al. Endobronchial treatment of giant emphysematous bullae with one-way valves: a new approach for surgically unfit patients. Eur J Cardiothorac Surg 2011;40:1425-31. [Crossref] [PubMed]
- Travaline JM, McKenna RJ, De Giacomo T, et al. Treatment of persistent pulmonary air leaks using endobronchial valves. Chest 2009;136:355-60. [Crossref] [PubMed]
- Anile M, Venuta F, De Giacomo T, et al. Treatment of persistent air leakage with endobronchial one-way valves. J Thorac Cardiovasc Surg 2006;132:711-2. [Crossref] [PubMed]
- Kesten S, Anderson JC, Tuck SA. Rationale for the development and mechanism of action of endoscopic thermal vapour ablation (InterVapor) for the treatment of emphysema. J Bronchology Interv Pulmonol 2012;19:237-45. [Crossref] [PubMed]
- Tuck SA, Lopes-Berkas V, Beam S, et al. Bronchoscopic thermal vapour ablation in a canine model of emphysema. Int J Chron Obstruct Pulmon Dis 2012;7:21-31. [Crossref] [PubMed]
- Emery MJ, Eveland RL, Eveland K, et al. Lung volume reduction by bronchoscopic administration of steam. Am J Resp Crit Care Med 2010;182:1282-91. [Crossref] [PubMed]
- Snell G, Herth FJ, Hopkins P, et al. Bronchoscopic thermal vapour ablation therapy in the management of heterogeneous emphysema. Eur Respir J 2012;39:1326-33. [Crossref] [PubMed]
- Gompelmann D, Eberhardt R, Schuhmann M, et al. Lung volume reduction with vapor ablation in the presence of incomplete fissures: 12-month results from the STEP-UP randomized controlled study. Respiration 2016;92:397-403. [Crossref] [PubMed]
- Herth FJ, Valipur A, Saha PL, et al. Segmental volume reduction using thermal vapour ablation in patients with severe emphysema: 6 month results of the multicenter, parallel-group, open-label, randomized controlled STEP-UP trial. Lancet Respir Med 2016;4:185-93. [Crossref] [PubMed]
- Shah PL, Gompelmann D, Valipur A, et al. Thermal vapor ablation to reduce segmental volume in patients with severe emphysema: STEP-UP 12 month results. Lancet Respir Med 2016;4:e44-e45. [Crossref] [PubMed]
- Klooster K, Ten Hacken NH, Slebos D. The lung volume reduction coil for the treatment of emphysema: a new therapy in development. Expert Rev Med Devices 2014;11:481-9. [Crossref] [PubMed]
- Hartman JE, Klooster K, Ten Hacken NH, et al. Treatment of emphysema using bronchoscopic lung volume reduction coil technology: an update on efficacy and safety. Ther Adv Respir Dis 2015;9:251-9. [Crossref] [PubMed]
- Herth FJ, Eberhard R, Gompelmann D, et al. Bronchoscopic lung volume reduction with a dedicated coil: a clinical pilot study. Ther Adv Respir Dis 2010;4:225-31. [Crossref] [PubMed]
- Slebos DJ, Klooster K, Ernst A, et al. Bronchoscopic lung volume reduction coil treatment of patients with severe heterogeneous emphysema. Chest 2012;142:574-82. [Crossref] [PubMed]
- Deslee G, Klooster K, Hetzel M, et al. Lung volume reduction coil treatment for patients with severe emphysema: a European multicenter trial. Thorax 2014;69:980-6. [Crossref] [PubMed]
- Shah PL, Zoumot Z, Bicknell S, et al. Endobronchial coils for the treatment of severe emphysema with hyperinflation (RESET): a randomized controlled trial. Lancet Respir Med 2013;1:233-40. [Crossref] [PubMed]
- Kontogianni K, Gerovasili V, Gompelmann D, et al. Effectiveness of endobronchial coil treatment for lung volume reduction in patients with severe heterogeneous emphysema and bilateral incomplete fissures: a six-month follow-up. Respiration 2014;88:52-60. [Crossref] [PubMed]
- Klooster K, Ten Hacken NH, Franz I, et al. Lung volume reduction coil treatment in chronic obstructive pulmonary disease patients with homogeneous emphysema: a prospective feasibility trial. Respiration 2014;88:116-25. [Crossref] [PubMed]
- Deslée G, Mal H, Dutau H, et al. Lung volume reduction coil treatment vs usual care in patients with severe emphysema. The REVOLENS randomized clinical trial. JAMA 2016;315:175-84. [Crossref] [PubMed]
- Hartman JE, Klooster K, Gortzak K, et al. Long-term follow-up after bronchoscopic lung volume reduction treatment with coils in patients with severe emphysema. Respirology 2015;20:319-26. [Crossref] [PubMed]
- Sciurba FC, Criner GJ, Strange C, et al. Effect of endobronchial coils vs usual care on exercise tolerance in patients with severe emphysema. The RENEW randomized clinical trial. JAMA 2016;315:2178-89. [Crossref] [PubMed]
- Reilly J, Washko G, Pinto-Plata V, et al. Biological lung volume reduction. A new bronchoscopic therapy for advanced emphysema. Chest 2007;131:1108-13. [Crossref] [PubMed]
- Mineshita M, Slebos DJ. Bronchoscopic interventions for chronic obstructive pulmonary disease. Respirology 2014;19:1126-37. [Crossref] [PubMed]
- Criner GJ, Pinto-Plata V, Strange C, et al. Biologic lung volume reduction in advanced upper lobe emphysema: phase 2 results. Am J Respir Crit Care Med 2009;179:791-8. [Crossref] [PubMed]
- Kramer MR, Refaely Y, Maimon N, et al. Bilateral endoscopic sealant lung volume reduction therapy for advanced emphysema. Chest 2012;142:1111-7. [Crossref] [PubMed]
- Come CE, Kramer MR, Dransfield MT, et al. A randomised trial of lung sealant versus medical therapy for advanced emphysema. Eur Respir J 2015;46:651-62. [Crossref] [PubMed]
- Slebos DJ, Klooster K, Koegelnberg CF, et al. Targeted lung denervation for moderate to severe COPD: a pilot study. Thorax 2015;70:411-9. [Crossref] [PubMed]
- van Geffen WH, Kertjens HAM, Slebos DJ. Emerging bronchoscopic treatments for chronic obstructive pulmonary disease. Pharmacol Ther 2017;179:96-101. [Crossref] [PubMed]
- Wang Y, Lai TW, Xu F, et al. Efficacy and safety of bronchoscopic lung volume reduction therapy in patients with severe emphysema: a meta-analysis of randomized controlled trials. Oncotarget 2017;8:78031-43. [Crossref] [PubMed]
- Valipour A, Herth F, Burghuber OC, et al. Target lobe volume reduction and COPD outcome measures after endobronchial valve therapy. Eur Respir J 2014;43:387-96. [Crossref] [PubMed]