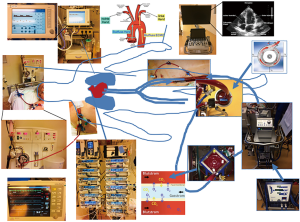
Monitoring of adult patient on venoarterial extracorporeal membrane oxygenation in intensive care medicine
Introduction
Extracorporeal cardiovascular support system or Extracorporeal Life Support System (ECLS) comprises a bridging therapy option covering acute cardiovascular and lung failure as well as elective cardio surgical interventions (1,2). In emergency cases, extracorporeal cardiopulmonary resuscitation (eCPR) can serve as an attempt at salvage for selected patients suffering from therapy-refractory cardiogenic failure with potentially reversible etiology (e.g., in terms of cardiogenic shock due to myocardial infarction). Owing to rapid technological and medical progress during the last years, numbers of patients with extracorporeal membrane oxygenation (ECMO) in cases of therapy-refractory cardiovascular or lung failure steadily increased (3). ECMO/ECLS-systems are complex intensive care bridging therapy devices needing intensive and high-quality monitoring in order to early detect possible severe complications and to manage them adequately (4,5). Use of ECLS provides hemodynamic support as well as oxygenation and decarboxylation of the blood. However, afterload will increase due to retrograde flow at the ascending aorta and aortic valve. An effective and balanced ECLS system only succeeds with an adequate controlling and intensive care monitoring.
This paper presents an overview for intensive care monitoring of veno-arterial ECMO (VA ECMO).
Definition
The term “ECMO“ refers by definition to veno-venous ECMO (VV ECMO). Thus, this term should only be used in this context. VA ECMO is equivalent with extracorporeal Life Support System (ECLS) (6).
VV ECMO is characterized by oxygenation as well as CO2-elimation of venous blood. Therefore, it is mainly used for isolated lung failure. An adequate cardiovascular support is not possible with this system (5). Use of ECLS enables not only gas exchange but also up to 80% of patient’s cardiac output (7).
The new term “mobile ECMO” is emerging. Offering patients possibility of being treated on road without losing valuable time. ECMO device can be implanted wherever patient is located and thus, patient can already be adequately treated on way back to hospital. This saves time and enhances patients’ survival rates in terms of improving neurological and hemodynamic outcomes.
Application of ECLS systems
In emergency cases, initiation of ECLS setting should only be carried out after careful consideration of all indications and contraindications. Further, case-to-case decision should be made by a skilled team (Table 1).
Implantation and components of ECLS systems
In an acute medical setting peripheral cannulation of inguinal vessels is preferred, also known as femo-femoral cannulation. Draining cannula is positioned via femoral vein (19–23 French) in the area of the right atrium and the back leading cannula is placed via femoral arteria in iliac arteria (15–19 French) (5). Due to cannulation strategy a right ventricular release is achieved as well as an adequate perfusion of visceral organs. The size of cannula determines amount of blood pump minute volume (PMV).
Besides peripheral femo-femoral cannulation, central surgical cannulation also exists. Thereby the draining cannula is surgically placed in the right atrium and the back leading cannula in the ascending aorta. Further, the possibility of a femo-subclavian technique is possible. In emergencies the last two mentioned methods are rarely used.
After successful implantation position of cannulas should be checked using echocardiography or radioscopy (X-ray or CT-scan). After implantation of femo-femoral ECLS system the implantation of an antegrade leg perfusion is obligate and cannulas are connected with ECLS system. The ECLS device consists of a blood pump (centrifugal pump) and a membrane oxygenator (Figure 1). Via a normothermia unit/recuperator, temperature can be managed. As the complete ECLS system, including cannulas, is coated, a strong indication for anticoagulation is given.
Case example—ECLS system
On a Saturday afternoon an emergency physician hands over a 55-year-old man with cardiogenic shock due to myocardial infarction. Sometimes before, the patient was collapsed buying cigarettes. After ten minutes of resuscitation, circulatory recovery could be achieved. At our university hospital a percutaneous coronary intervention (PCI) was performed with stenting of the proximal left anterior descending (LAD) artery. However, left ventricular ejection fraction was seriously reduced and patient needed highly dosed catecholamines, and based on this situation the heart team decided to implant an ECLS system.
After successful PCI an ECLS was implanted via femo-femoral access. On intensive care unit diuresis suspended, so that the patient went on dialysis. In terms of adequate anticoagulation, activated clotting time was measured every hour. Further, antegrade leg perfusion was hourly checked using Doppler ultrasonography as well as function of heart circulation via echocardiography. Catecholamines and volume were also controlled and optimized. The hemodynamic unstable patient with reduced ejection fraction was deeply sedated. Oxygenation was measured by blood gas analysis via right radial arteria. Patient was monitored 24/7 by skilled caregivers in a 1:1 supervision.
Intensive care monitoring under ECLS
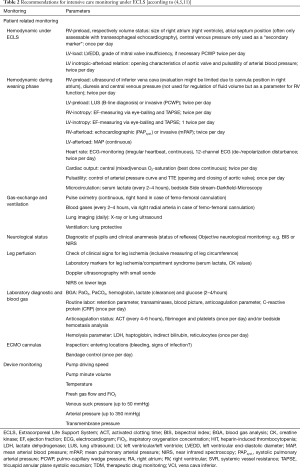
Currently a German S3-guideline for use of ECLS/ECMO for cardiac or lung failure as well as a consensus paper for ECMO is in progress (8). Recommendations for ECLS monitoring predominantly originate from general recommendations of “Extracorporeal Life Support Organization” (ELSO) [ELSO 1–4, 2017 (5,9)], reviews (7,10) and position papers (4,11). Up to now autonomous recommendation for monitoring of ECLS does not exist despite wide distribution of ECLS systems. After successful ECLS implantation a continuous intensive care monitoring is necessary (Tables 2,3).
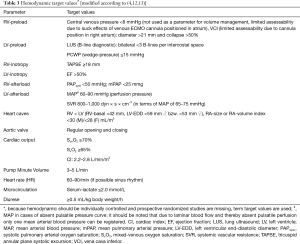
Despite classic hemodynamic monitoring it should be differentiated among preload, inotropy and afterload under ECLS therapy conditions (preload-dependent and afterload-sensible). Mainly volume status, status of left ventricle release and the left ventricular inotropy-afterload relation is important. These parameters are of high relevance for regulation management (volume loading or additional left-ventricular unloading). During weaning phase classic hemodynamic parameters should be used.
Management of ECLS patients needs a special staff-intensive monitoring. Care-giver-patient ratio should be 1:1. Care-giver should be well trained with a high level of knowledge in ECLS management (4,14).
Hemodynamic, catecholamine therapy and perfusion flow
Non-invasive (focused echocardiography) as well as invasive (pulmonary artery catheter) hemodynamic monitoring are obligatory for patients under ECLS (Table 3) (4). Measurement of cardiac output by means of thermodilution method and measurement of central (mixed) venous oxygen saturation are often overestimated because venous blood arriving in right atrium is mainly withdrawn by the therein located venous cannula. Further, cardiac output measurement methods based on pulse contour analysis should not be used when pulsatile dynamic is absent. In cases of absent native cardiac output a non-pulsatile arterial pressure curve, an absent opening and closing performance of aortic valve as well as absent heart contraction can be detected. Due to these reasons echocardiography gained importance for hemodynamic monitoring (15,16).
In cases of absent myocardial contractility, ECLS adopts on cardiovascular and lung function, i.e., oxygenation is not delivered by means of ventilation (stasis in lung circulation) but by gas flow and FDO2 (fraction of delivered oxygen).
In context of an acute cardiac output failure, necessity of an additional catecholamine therapy often exists. It should be mentioned that catecholamine therapy should only be employed after consideration of volume status. Since for patients under ECLS inotropy is managed by a “machine”, regulation of volume status and afterload through vasopressors is paramount. During weaning phase supportive inotropes should be used. As inotropes dobutamine, phosphodiesterase-inhibitors (e.g., milrinon) and on the other hand levosimendan are available. Dobutamine is often used and is associated with a cardio depression in case of left ventricular dysfunction, increase of myocardial oxygen consumption and proarrhythmia (17). Levosimendan proved advantageous in comparison to other inotropic drugs regarding weaning of ECLS patients (18). Since catecholamine therapy is not only associated with higher side effects but also with higher mortality, these drugs should only be used in a restricted way.
Patients under ECLS need a special volume management. Inadequate volume status offers danger of suction of ECMO cannulas on vessel wall and thus ECMO output (low-flow alarm) decreases. Since modern centrifugal pumps are preload-dependent and afterload-sensible, a preload monitoring should be checked several times per day. Ultrasound evaluation of inferior caval vein in order to measure volume dynamics is limited due to position of ECMO cannula in the right atrium so that echocardiographic evaluation is a good method to evaluate inflation condition of all four heart chambers in combination with lung ultrasound (B-line diagnostic) (15).
Beside perfusion “pressure” (MAP), also perfusion “flow” plays a crucial role for an adequate organ perfusion and microcirculation. Perfusion flow or pump minute volume are determined by the following formula: flow = body surface (m2) × cardiac index (L/min/m2). Thus, perfusion flow depends on body surface and cardiac index about 3 to 5 liters per minute. Control of perfusion flow should be done together with volume management and necessary catecholamine therapy. MAP is regulated by perfusion flow and total peripheral vascular resistance. Reduction of PMV depending on PVR leads to decrease of MAP and vice versa. An inadequate pump minute volume needs an evaluation of hemodynamic status being analyzed by focused bedside echocardiography (15).
By means of echocardiography not only hemodynamic status but also reasons for decrease of PMV (pericardial effusion) or complications (left ventricular thrombus) under ECLS can be evaluated (16). Daily evaluation of left and right ventricular pump function as well as size of all four heart chambers belongs to standard hemodynamic monitoring of ECLS patients (10). Further, evaluation of aortic valve opening characteristics is important. Since under femo-femoral ECLS retrograde aortic flow competes against antegrade ejected stroke volume, a closed aortic valve might result in cases of a minimal or missing left ventricular contraction and full ECMO power. A closed aortic valve may lead to a left ventricular dilatation with a consecutive functional mitral valve insufficiency und pulmonary back pressure as well as to the danger of left ventricular thrombus development so that in order to unload left ventricle and decrease of left ventricular preload (LVEDP) an implantation of a percutaneous cardiac support system, e.g., a left ventricular micro-axial-pump or a venting system, should be considered (19). If myocardial contraction increases during ECLS, visible by a better pump function and a more regularly opening of the aortic valve, ECLS flow rate can slowly be reduced depending on hemodynamic and clinical status.
Gas exchange and ventilation
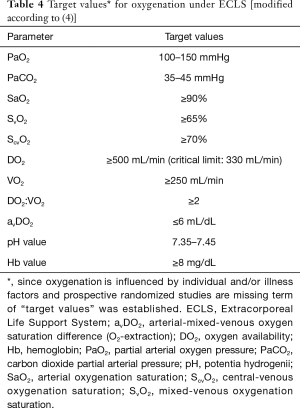
Monitoring of gas exchange should always be regarded in combination with hemodynamic status of the patient, since oxygen supply as well as oxygen consumption depend on cardiac output volume and pump minute volume. In order to avoid tissue hypoxia, ratio of DO2 to VO2 should be above 2, if possible.
Oxygen supply (DO2) is product of cardiac output multiplied with arterial oxygen content (CaO2). In terms of ECLS setting, following relation can be derived: DO2 = (HZVnative × CaO2Lung, native [right arteria]) + (PMVECMO × CaO2membrane oxygenator). Arterial oxygen content (CaO2) depends on arterial oxygen saturation (SaO2) and of hemoglobin value CaO2 = SaO2 × 1.34 × Hb). Oxygen consumption (VO2) is calculated out of cardiac output multiplied with arterio-mixed-venous oxygen content difference (avDO2). Arterio-mixed-venous oxygen content difference (avDO2) is difference of arterial and mix-venous oxygen content. In order to measure mixed-venous oxygen saturation a pulmonary artery catheter is necessary. However, due to the fact that the arriving venous blood in right atrium is mainly absorbed by the venous ECMO cannula, alternatively central venous oxygen saturation can be measured. Oxygen binding is additionally modulated by metabolic factors. A complicated oxygen release to tissue occurs in cases of hypothermia, acidosis, hypercapnia or hyperkalemia.
Under ECLS therapy, complexity of O2-physiology is complicated by the fact that oxygenation, which depends on the contractility of heart, is managed either via ventilation in cases of own cardiac output or via ECMO console in cases of missing pulsatility. In cases of residual heart contractility, with regard to peripheral ECLS cannulation, oxygen-deficient blood is ejected by the left ventricle and mixed with oxygen-enriched blood ejected of “antegrade ECMO circuit”. The location of mixture, so-called “watershed”, depends on the extent of left ventricular ejection, retrograde blood flow of ECLS and on arterial resistance (MAP). In case of location of meant “watershed” in distal aortic arch, hypoxia of upper body area results in myocardial and cerebral hypoxia (PaO2 Aorta ascendens < PaO2 Aorta descendens). In cases of additional lung pathology e.g., ventilation-associated pneumonia an oxygenation impairment develops so that in cases of inadequate pulmonary gas exchange O2-partial pressure of left-ventricular blood further decreases. Because the patient is supplied with oxygen via ECLS as well as via invasive ventilation system the art of ECLS intensive care team is to guarantee a balanced oxygenation in order to avoid myocardial ischemia as well as cerebral hypoxia. In case of femo-femoral cannulation blood gas analysis should be conducted via an arterial access in the right brachial artery in order to estimate native oxygenation and early detection of hypoxia in upper body area, so-called “Harlequin syndrome”. This phenomenon occurs quite often during weaning phase.
Furthermore, in cases of a missing pulsatility, a valid measurement of SpO2 is difficult and thus regular blood gas controls are very important (Table 4). Besides regular blood gas analyses cerebral monitoring can be conducted using a NIRS (near infrared spectroscopy) system (20).
In case of “Harlequin syndrome”, e.g., due to combined cardiac and respiratory failure, ECLS can be combined with VV ECMO, called veno-arterial-venous ECMO (VAV ECMO) (21). For this purpose a second venous cannula is placed via right internal jugular vein into superior caval (VCS) vein. Afterwards arterial supply is so modified via a y-connection so that oxygen-saturated blood is delivered via VCS cannula as well as via cannula in iliac arteria. Venous sucking cannula should be withdrawn up to inferior caval vein in order to avoid recirculation.
With regard to ventilation under ECLS, basic principles of lung protective ventilation should be followed such as pressure-controlled ventilation with an ideal positive end-expiratory pressure (PEEP), low tidal volume (≤6 mL/kg), moderate plateau pressure (≤30 mbar) and a low driving-pressure (<15 mbar) according to recommendations for ventilation strategy of VV ECMO (11,12).
Neurological status
Particularly with regard to obligatory anticoagulation with the risk of cerebral bleeding or stroke or development of Harlequin syndrome as well as evaluation of sedation depth, an adequate neurological monitoring is obligatory (22). Frequent controls of pupils of sedated patients are crucial in order to early detect a neurological event. In cases of ECLS in conscious patients cloudinesses, lalopathies or new weaknesses in extremities are possible signs of a neurological event. Whether device-assisted methods such as e.g., BIS (bispectral index)- or NIRS (near infrared spectroscopy)-monitoring support or replace subjective evaluation using RASS (Richmond agitation-sedation scale)-Scoring will be matter of future study (23). A standardized neurological monitoring of ECLS patients does not exist until today.
Leg perfusion
Due to femo-femoral cannulation an antegrade leg perfusion cannula in the superficial femoral arteria should be inserted in order to avoid ischemia of the arterial cannulated extremity. After implantation of leg cannula an imaging diagnosis (Doppler ultrasonography or CT-scan) is recommended in order to check position of the cannula. After successful femo-femoral cannulation with insertion of an antegrade leg perfusion cannula, this leg cannula should be checked every 2 to 4 hours (inspection, palpation, measurement of leg circumference) as well as via Doppler ultrasonography (arteria tibialis posterior and arteria dorsalis pedis). Rule of thumb states that a warm leg is usually perfused. In cases of a marbled leg without a Doppler sound acute leg ischemia must be suspected and then vascular surgeon should be called. Also, increasing lactate values might indicate ischemia. Besides clinical Doppler ultrasonography and laboratory controls in order to detect leg ischemia, measurement of regional oxygenation saturation using NIRS technology has proved efficacy (24).
Anticoagulation and blood management
The ECLS patient needs an obligate anticoagulation regime due to procoagulant activity of the foreign surface area of the ECMO system. On the other hand a high bleeding tendency should be prevented. Anticoagulation is implemented using unfractionated heparin (10 to 70 IU/kg body weight/h i.v.) or in case of heparin-induced thrombocytopenia heparin substitute products such as argatroban are used.
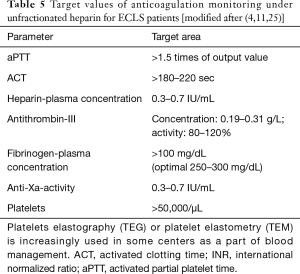
Hourly anticoagulation checks [Measurement of ACT (activated clotting time) and PTT (partial thromboplastin time)] and a standardized neurological monitoring (danger of central-located bleeding) are indispensable (Table 5). Bedside anticoagulation monitoring and classic anticoagulation controls in laboratory play an important role. Point-of-Care (POC) methods enable bedside ACT- as well as PTT-controls for ECLS patients (25). In contrast to ACT controls, POC-based PTT measurement is not identical with PTT analyzed in laboratory. Further checks such as measurement of heparin or antithrombin concentrations are often additionally necessary.
Full table
Since until today no common consensus in terms of anticoagulation management under ECLS exists, establishment of an internal standard including blood management is recommended for each center using ECLS. For further information of management of blood complications, the following literature is recommended (26,27).
Pharmacokinetic aspects
Critically ill patients usually suffer from modified pharmacokinetics. Besides end-organ dysfunctions (kidney and liver insufficiencies) with reduced elimination and clearance or hypoalbuminemia leading to reduced plasma protein binding capacity of certain medical drugs (e.g., antibiotics), a higher distribution volume (especially for hydrophilic pharmaceutics) and medical drug interactions with ECMO device materials must be considered (28). In cases of non-response to anti-infective drugs such as oseltamivir, rifampicin und voriconazol a therapeutic drug monitoring for ECLS patients should be followed. Future randomized controlled PK-studies are necessary for ECLS patients in order to establish optimal dosage guidelines. Since not every hospital has a TDM-laboratory a clinical-pharmacological cooperation with an external institute should be initiated.
Case example—ECMO weaning
Patient’s cardiovascular situation steadily improved. High-dose inotropic therapy could be reduced as well as sedation. Patient awoke 7 days after ECLS implantation and was extubated. Initially overstrained with the situation, the patient stabilized. ECLS weaning was conducted. In regular echocardiography examinations an ejection fraction of 45% was observed, so that ECMO flow was reduced and finally ECLS was explanted. Patient was released to rehabilitation facility 14 days after his myocardial infarction. After three further weeks he was released home to his family. He stopped smoking.
Complications under ECLS
Under ECLS technical as well as non-technical complications can occur. In terms of technical complications failure of oxygenator or power may happen. In case of power failure a battery supplies energy for about 30 to 60 minutes. When this period is extended, ECLS pump can be cranked by hand. In terms of non-technical complications cannula associated problems, intracerebral bleeding, infections, or pump thrombosis can happen as well as leg ischemia with consecutive compartment syndrome. In general, possible complications should preventively be avoided by well-structured education and trainee programs, security instructions, “time-out” meetings and post-ECLS analysis. Further complication management options are described in detail in literature (29,30).
Summary
ECLS as a cardiopulmonary bypass is a cardiovascular and lung support system. However it is not a causal therapy. Monitoring of patients under ECLS comprises time-consuming and staff-intensive management. Besides intensive care monitoring, special aspects of ECLS should be checked regularly and optimized. With regard to patient-side monitoring control of hemodynamics, gas exchange, anticoagulation status, leg perfusion as well as neurological monitoring is very important. In terms of device monitoring pump flow per minute, fresh gas flow and inspiratory O2-fraction have to be regularly documented. A multi-professional and interdisciplinary team of caretakers and physicians well-skilled in use of ECLS system is indispensable of qualitative and patient-safe ECLS setting.
Key points
- Until today autonomous recommendation for ECLS monitoring in form of guidelines or position papers does not exist.
- Management of ECLS can be divided in patient- and device-related monitoring.
- Staff-intensive care of ECLS patients is necessary in order to detect and mange severe complications.
- In terms of hemodynamic monitoring, bedside-focused echocardiography is of high significance.
- In order to avoid Harlequin syndrome in case of femo-femoral cannulation, blood gas analysis should only be conducted via an arterial access in the right arm.
ECLS technique should only be conducted in hospitals with an ECMO/ECLS program and with supply of skilled personal since these patients need an interdisciplinary care management structure.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kulkarni T, Sharma NS, Diaz-Guzman E. Extracorporeal membrane oxygenation in adults: A practical guide for internists. Cleve Clin J Med 2016;83:373-84. [Crossref] [PubMed]
- Abrams D, Garan AR, Abdelbary A, et al. Position paper for the organization of ECMO programs for cardiac failure in adults. Intensive Care Med 2018;44:717-29. [Crossref] [PubMed]
- Karagiannidis C, Brodie D, Strassmann S, et al. Extracorporeal membrane oxygenation: evolving epidemiology and mortality. Intensive Care Med 2016;42:889-96. [Crossref] [PubMed]
- Beckmann A, Benk C, Beyersdorf F, et al. Position article for the use of extracorporeal life support in adult patients. Eur J Cardiothorac Surg 2011;40:676-80. [PubMed]
- ELSO Guideline for Cardiopulmonary Extracorporeal Support Extracorporeal Life Support Organization Extracorporeal Life Support Organization. August 2017 (Version 1.4).
- Trummer G, Bein B, Buerke M, et al. Standardized terminology of mechanical heart, lung and circulatory assist devices: A recommendation of the Section “Heart and Circulation” of the German Interdisciplinary Association of Critical Care Medicine. Applied Cardiopulmonary Pathophysiology 2011;15:181-2.
- Chung M, Shiloh AL, Carlese A. Monitoring of the adult patient on venoarterial extracorporeal membrane oxygenation. ScientificWorldJournal 2014;2014:393258. [Crossref] [PubMed]
- Invasive beatmung und Einsatz extrakorporaler Verfahren bei akuter respiratorischer Insuffizienz. AWMF Leitlienen, 2017.
- . Available online: www.elso.orgKriterien ELSO.
- Fitzgerald DC, Darling EM, Cardona MF. Staffing, Equipment, Monitoring Considerations for Extracorporeal Membrane Oxygenation. Crit Care Clin 2017;33:863-81. [Crossref] [PubMed]
- Pichler P, Antretter H, Dunser M, et al. Use of ECMO in adult patients with cardiogenic shock: a position paper of the Austrian Society of Cardiology. Med Klin Intensivmed Notfmed 2015;110:407-20. [Crossref] [PubMed]
- Werdan KRM, Buerke M, Engelmann L, et al. Deutsch-österreichische S3-Leitlinie Infarktbedingter kardiogener Shock. Diagnose, Monitoring und Therapie. Der Kardiologe 2011;5:166-224. [Crossref]
- Galderisi M, Cosyns B, Edvardsen T, et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: an expert consensus document of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2017;18:1301-10. [Crossref] [PubMed]
- Empfehlungen zur Struktur und Ausstattung von Intensivstationen. Available online: https://www.divi.de/empfehlungen/publikationen/intensivmedizin/399-empfehlungen-zur-struktur-von-intensivstationen-langversion/file
- Price S, Platz E, Cullen L, et al. Expert consensus document: Echocardiography and lung ultrasonography for the assessment and management of acute heart failure. Nat Rev Cardiol 2017;14:427-40. [Crossref] [PubMed]
- Douflé G, Roscoe A, Billia F, et al. Echocardiography for adult patients supported with extracorporeal membrane oxygenation. Crit Care 2015;19:326. [Crossref] [PubMed]
- Abraham WT, Adams KF, Fonarow GC, et al. In-hospital mortality in patients with acute decompensated heart failure requiring intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE). J Am Coll Cardiol 2005;46:57-64. [Crossref] [PubMed]
- Sangalli F, Avalli L, Laratta M, et al. Effects of Levosimendan on Endothelial Function and Hemodynamics During Weaning From Veno-Arterial Extracorporeal Life Support. J Cardiothorac Vasc Anesth 2016;30:1449-53. [Crossref] [PubMed]
- Spartera M, Jabbour RJ, Chiarito M, et al. Stepwise use of circulatory support devices in a patient refractory to cardiopulmonary resuscitation. Cardiovasc Revasc Med 2017;18:447-9. [Crossref] [PubMed]
- Pozzebon S, Ortiz AB, Franchi F, et al. Cerebral Near-Infrared Spectroscopy in Adult Patients Undergoing Veno-Arterial Extracorporeal Membrane Oxygenation. Neurocrit Care 2018;29:94-104. [Crossref] [PubMed]
- Cakici M, Gumus F, Ozcinar E, et al. Controlled flow diversion in hybrid venoarterial-venous extracorporeal membrane oxygenation. Interact Cardiovasc Thorac Surg 2018;26:112-8. [Crossref] [PubMed]
- Lorusso R, Taccone FS, Belliato M, et al. Brain monitoring in adult and pediatric ECMO patients: the importance of early and late assessments. Minerva Anestesiol 2017;83:1061-74. [PubMed]
- DAS-Taskforce 2015, Baron R, Binder A, et al. Evidence and consensus based guideline for the management of delirium, analgesia, and sedation in intensive care medicine. Revision 2015 (DAS-Guideline 2015) - short version. Ger Med Sci 2015;13:Doc19. [PubMed]
- Werner T, Lunz D, Philipp A, et al. Use of near-infrared spectroscopy for control of limb perfusion during venoarterial ECMO treatment: Application and limitations. Anaesthesist 2017;66:862-6. [Crossref] [PubMed]
- Bolliger D, Zenklusen U, Tanaka KA. Point-of-care coagulation management algorithms during ECMO support: are we there yet? Minerva Anestesiol 2016;82:1000-9. [PubMed]
- Görlinger K, Bergmann L, Dirkmann D. Coagulation management in patients undergoing mechanical circulatory support. Best Pract Res Clin Anaesthesiol 2012;26:179-98. [Crossref] [PubMed]
- Thomas J, Kostousov V, Teruya J. Bleeding and Thrombotic Complications in the Use of Extracorporeal Membrane Oxygenation. Semin Thromb Hemost 2018;44:20-9. [Crossref] [PubMed]
- Hahn J, Choi JH, Chang MJ. Pharmacokinetic changes of antibiotic, antiviral, antituberculosis and antifungal agents during extracorporeal membrane oxygenation in critically ill adult patients. J Clin Pharm Ther 2017;42:661-71. [Crossref] [PubMed]
- Meuwese CL, Ramjankhan FZ, Braithwaite SA, et al. Extracorporeal life support in cardiogenic shock: indications and management in current practice. Neth Heart J 2018;26:58-66. [Crossref] [PubMed]
- Le Gall A, Follin A, Cholley B, et al. Veno-arterial-ECMO in the intensive care unit: From technical aspects to clinical practice. Anaesth Crit Care Pain Med 2018;37:259-68. [Crossref] [PubMed]