Robotic thoracic surgery: from the perspectives of European chest surgeons
Video-assisted thoracoscopic surgery (VATS) has been a strong alternative to thoracotomy for lobectomy in patients with early stage lung cancer. The success of improved endoscopic video systems and endoscopic staplers has increased the thoracic surgeons’ capabilities to perform complicated thoracic procedures since 2000. In the current era, the world wide experience with VATS resections for lung cancer is sufficiently large to compare the outcome with open thoracotomy, which was unforeseen in 1993 by an experienced author of the North America (1). Miller predicted that VATS would be a tool to be used in 25-30% of all activities of an active, general thoracic surgeon’s practice. More than this, he did not believe lung cancer surgery could have ever been a common indication for VATS.
In 2008, a comprehensive and methdological review and survey demonstrated that VATS lobectomy was not a commonly used procedure among European surgeons, with a rate of not more than 5% using the VATS technique among the surgeons who filled out the survey (2). Although in current practice, there are several European thoracic surgery clinics performing VATS lobectomy at a rate higher than 50% in all lung cancer patients (personal communications). However, there is still a lack of adoption of the technique. This may be attributed to several factors, including a lack of oncological control by means of lymph node dissection and experience, and limitations in instrumentation and depth sensation. In addition to the above mentioned concerns, a fear of hemorrhage and an inability to control the bleeding has made thoracic surgeons hesitate to adopt the minimally invasive lobectomy. All of these have occurred within the past two decades.
To overcome these limitations in minimally invasive resections, robotic surgery has been designed. With the development of the surgical robot (Intuitive, Da Vinci, Inc, Sunnyvale, CA, USA), the performance of urologic, gynecologic and cardiac operations has been proven to be safe and feasible. Robotic thoracic surgery reports were presented within the past decade very rarely (3-6). Several European countries—Italy, France, Austria, Germany, Switzerland and Belgium—lead the development of robotic surgery in the world, especially Italy for lung cancer surgery and Germany for thymus—thymoma surgery. This manuscript describes the development of a robotic thoracic surgery program in the context of Europe.
European surgeons and their contributions to robotic surgery platform
Several European thoracic surgery centers did important contributions to Robotic thoracic surgery. University of Pisa was the first to perform a robotic lobectomy in Europe with da Vinci Robotic Systems in February 2001 and published this initial experience in 2002 (3). They summarized their robotic lobectomy experience in 2008 on 107 good-risk patients. They reported that all their patients returned to preoperative levels of physical activity within 10 days (7). From November 2006 through September 2008, 54 patients with suspected or proven clinical stage 1 or 2 lung cancer were recruited to undergo robotic lobectomy. Veronesi was the sole surgeon to perform these lobectomies in several European centers. She concluded that the robotic lobectomy with lymph node dissection is practicable, safe and associated with shorter postoperative hospitalization than open surgery. She found that the robotically dissected mediastinal lymph nodes were similar in number to those of open surgery and robotic lobectomy could be applied to early lung cancer treatment (8). In 2013, a large robotic thymectomy series was published by the University of Padua. Authors described the robotic thymectomy technique as a safe and effective procedure. They observed a neurological benefit in great number of patients and a better clinical outcome was obtained in patients with early stages of clinical conditions (9). Four European centers collected their data on robotic thymoma resections. They analyzed 79 patients with early stage thymoma who were operated on between 2002 and 2011. They indicated that the robotic enhanced thoracoscopic thymectomy for early stage thymoma was a technically sound and safe procedure with a low complication rate and short hospital stay (10). The oncologic outcomes seemed good (10).
VATS and robotics and VATS versus robotics
A lobectomy with systematic mediastinal lymph node dissection remains the “gold standard” for the treatment of early-stage NSCLC (11). Although this concept was already accepted during the era of open thoracotomies, lobectomies with VATS continues to be questioned. With the advancement of minimally invasive surgery, many surgeons have developed capabilities to perform lobectomy with VATS. After a decade of collecting data on VATS lobectomies, comparisons of open versus VATS have become available. When a VATS lobectomy is compared with that of thoracotomy, VATS is shown to have a decreased hospital stay, an improved postoperative pulmonary function, decreased pain, and a lower morbidity (12-14). However, concerns remain over the oncological principles of lung cancer surgery and VATS’ ability to respect them. The published research favors the abovementioned benefits of the new technology VATS over the open approach (15). Finally, the survival data establishes that VATS is at least equivalent to thoracotomy for the early-stage of NSCLC. Despite the development of new instrumentation for the VATS approach, the standardization of the VATS technique, and the superior outcomes of VATS, a review of the STS database shows a limited adoption (16). Yet, due to the challenges of learning and practicing the techniques, we do not have enough evidence to say that VATS is the “standard-of-care” for the treatment of early stage lung cancer.
Although both VATS and robotics are minimally invasive techniques using a comparable number of ports, there tends to be a comparison or split of the data. While robotic surgeons site VATS’ results and benefits, VATS surgeons often deny the similarities, instead demanding the original data provided by the robotic surgeons. As there are not many reports on robotic lung cancer surgeries, it is too early to know and compare the long term survival rates. Recently published reports suggest that there may be certain advantages of the robotic approach over VATS. It is suggested that the robotic surgery offers better instruments and a better view of the operative field: 3-dimensional rather than 2-dimensional; 10× magnification rather than 2× or 3×; and less fogging, therefore less camera manipulation required. Most surgeons who passionately try to learn both the VATS and robotic techniques agree that the robot provides clear advantages for mediastinal and esophageal operations (17). The advantages for robotic lung surgery may include better dissection of enlarged or metastatic N1 lymph nodes off the pulmonary artery, more precise and thorough N2 lymph node dissection, and less operative blood loss. The robot may be less painful than VATS and leads to fewer conversions. However, there are no reports that clearly support these “advantages” and improved outcomes for robotic resections.
There are several large series of lung cancer resection. The robotic group had a reduced morbidity, a lower mortality, an improved mental health, and a shorter hospital stay when comparing the 106 patients who had a lobectomy with robotic surgery with the 318 propensity-matched patients who underwent lobectomy via nerve and rib-sparing thoracotomy (17). According to the author of this paper, robotic surgery is clearly superior to the open approach. Therefore, the concern is not that robotic surgery is superior to the open approach, but if there are any superiorities to the VATS technique.
Swanson and co-workers analyzed the STS data to compare the VATS to robotics. The results indicate that robotic lobectomy and wedge resection seem to have higher hospital costs and longer operating times, without any differences in the adverse events (18). This study shows some noteworthy limitations (18). These include the lack of preoperative data—patient body mass index and smoking habits—and postoperative data—pain scores, quality of life, morbidity, and time to return to work. Furthermore, intraoperative data regarding the precision of surgery—the surgical margins, the adequacy of lymph node dissection, the amount of bleeding, and adverse events during surgery—were not evaluated.
Results of robotic lung cancer surgery
Previous reports demonstrate the safety of robotic pulmonary resections (19,20). Veronesi and associates from Milan report the safety of a 4-arm robotically assisted (not completely portal) lobectomy (with a 3- to 4-cm access incision, such as the one used by VATS surgeons) in 54 patients (8). Ninan and coworkers report the effectiveness of a completely portal 3-arm robotic lobectomy in 74 patients (19). Another study by the same group reports that robotic video-assisted pulmonary resection was accomplished in 197 of 200 patients: a total of 154 patients underwent lobectomy; 4 patients required bilobectomy, and 35 patients underwent segmentectomy. One patient received a left pneumonectomy. Three patients required conversion to a thoracotomy. The median operative time was 90 minutes. The median length of hospital stay was 3 days. The 60-day mortality and morbidity rates were 2% and 26%, respectively. Robotic VATS (RVATS, as the group names the technique) lung resection is technically feasible and safe. Their results indicate that the procedure is associated with a reduced length of stay, and a low morbidity and mortality (20). Our operative results and complications show similarities with this report.
One of the most influential manuscripts presented the long term outcomes of 325 robotic lobectomy patients who were operated on at three thoracic surgery centers (two from Italy and one from the US) from 2002 to 2010 (21). They concluded that the robotic lobectomy was a safe procedure for early stage lung cancer patients. The long term stage specific survial was acceptable and consistent with prior results for VATS and thoracotomy (21).
Learning, education and future perspectives
There are two recently published papers questioning the transition from VATS to robotics.
The second paper evaluates an established VATS single surgeon’s learning curve in a robotic lobectomy program (22). This retrospective review was conducted on patients undergoing minimally invasive lobectomy (robotics or VATS) for lung cancer. It concludes that, based on the clinical outcomes, there does not seem to be a significant advantage for an established VATS lobectomy surgeon to transition to robotics. The learning curve for robotic upper lobectomies seems to be significantly more difficult than that for lower lobectomies (22). Although our program demonstrates similarities in terms of starting a robotic thoracic program after an established VATS program, we don’t share the conclusions given in this paper. We believe the advancement of the technology brings superior health care. Today we may not recognize these differences as they happened during the initial development of VATS. Today, we may not yet provide the data necessary to demonstrate the superiority of the robotic technology over VATS. But the next generation of surgeons, with their enthusiasm and computer-based capabilities, will decide. Forecasting the future trends, one may clearly see that standardization in surgical education may only be provided through computer-based systems, rather than the classical Halstedian learning systems (see one—do one—teach one). The apprenticeship style of learning may fade away within two decades. Instead, the next generation may rely on simulators, learning through simulation rather than on patients; they may even be recognized and certified as surgeons by the computer-enhanced accreditation systems. Even today, simulators and robots have the capability to differentiate an expert from a novice (23). In this study, the authors describe an open-ended longitudinal study and automated motion recognition system capable of objectively differentiating between the clinical and technical, operational skills in robotic surgery.
The robot measures and collects data on the skill paramaters of the trainees operating it. As the novices gain practice during the training protocols, their results, measured by the robot, converge to be the same as those of expert robotic surgeons (23).
The robotic technology may bring new surgical educational standards worldwide. Through the standardization of these techniques, patients may be operated on in a standard way around the world. The computer enhanced programs may allow monitoring of the quality of surgery. Telesurgical apprenticeship or assistance may be provided to those who need mentorship or assistance during a particular surgery. Yet we may not have the data to prove the clear benefits of robotic surgery unless more surgeons adopt the techniques.
From the discussion, it is clear that European chest surgeons credited robotic thoracic surgery and created the most of the literature and the data behind it. We believe that robotic thoracic surgery will be developed by the enthusiastic chest surgeons all around the world. The European Society of Chest Surgeons will start to organize robotic surgery courses and will help dissemination of the knowledge in the upcoming years.
Robotic surgery experience in our center
We started our thoracic robotic program after an established experience of VATS surgery program. Our VATS program included >300 anatomical lung resections and >350 thymectomies and >60 thymomectomies. The idea of the start of a thoracic robotic program relied on the difficulties of some anatomical VATS lung resections. Here, in this manuscript, we presented our experience in the first 29 months of experience. We still continue to perform VATS anatomical resections for lung cancer and other pathologies, which may enable comparative studies in the upcoming years. Our case series demonstrates a nice distribution among pathologies and type of operations. This may provide the evidence of similarities with VATS abilities. We may also claim that the rate of segmentectomy is relatively higher when compared to lobectomy, which may be a sign that the robot could be used for even more precise dissection of small vessels and bronchi.
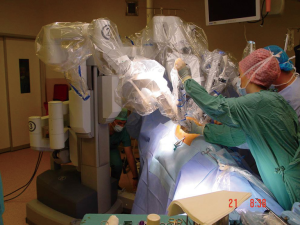
Between October 2011 and March 2014, 87 consecutive patients (25 females and 62 males) underwent a robotic assisted thoracic surgery. We preferred docking from superior and posterior to the patient in all lung resections (Figure 1). The patient characteristics are listed in Table 1. Thirty-five patients underwent an anatomical lobectomy. Only two patients underwent lobectomy for benign lesions: one patient with bronchiectasis and one patient with pulmonary aspergilloma. All other patients were operated on for lung cancer. Four patients had a neoadjuvant treatment due to single node N2 disease prior to the scheduled robotic operations. Two patients underwent left pneumonectomy, one patient for invasive N1 lymph node, and the other one for a hilar located, sleeve impossible lesion.
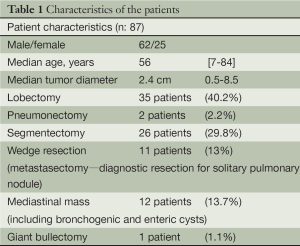
Full table
Twenty-six patients were operated on using formal segmentectomies: 13 from the right lung and 13 from the left lung. Eleven patients had a segmentectomy from the upper lobes and 15 patients from the lower lobes. The mean duration of chest tube drainage and postoperative hospital stay were 3±3.1 [1-10] days and 4±1.8 [2-7] days, respectively. Out of 74 lung resection operations, four patients required conversions to a muscle-sparing mini-thoracotomy due to bleeding (two patients) and difficulties (two patients). In our series, upper-lobe NSCLC lesions predominated, with the right upper lobe being the most common tumor site.
No patient required an epidural catheter for postoperative pain control. The median length of stay in the intensive care unit (ICU) was 1 (range, 0-1) day. The complication rate for the study cohort was 20 out 87 patients (Table 2). Most complications occurred in patients who underwent a lobectomy (9/35). The most common complications were air leaks for more than five days (five patients) and atrial fibrilation (three patients). One patient died within 30 days of the operation; he was discharged after a right upper lobectomy for squamous cell lung cancer. He was readmitted one week later with an infiltration of the contralateral lung and leucocytosis of 88.000/mL. He was diagnosed with a concurrent lymphoblastic lymphoma through the bone marrow aspiration biopsy, and died of chemotherapy side effects.
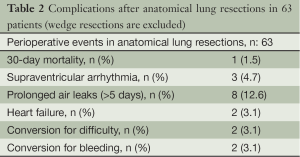
Full table
Acknowledgements
Disclosure: The author declares no conflict of interest.
References
- Miller JI Jr. The present role and future considerations of video-assisted thoracoscopy in general thoracic surgery. Ann Thorac Surg 1993;56:804-6. [PubMed]
- Rocco G, Internullo E, Cassivi SD, et al. The variability of practice in minimally invasive thoracic surgery for pulmonary resections. Thorac Surg Clin 2008;18:235-47. [PubMed]
- Melfi FM, Menconi GF, Mariani AM, et al. Early experience with robotic technology for thoracoscopic surgery. Eur J Cardiothorac Surg 2002;21:864-8. [PubMed]
- Bodner J, Wykypiel H, Wetscher G, et al. First experiences with the da Vinci operating robot in thoracic surgery. Eur J Cardiothorac Surg 2004;25:844-51. [PubMed]
- Park BJ, Flores RM, Rusch VW. Robotic assistance for video-assisted thoracic surgical lobectomy: technique and initial results. J Thorac Cardiovasc Surg 2006;131:54-9. [PubMed]
- Gharagozloo F, Margolis M, Tempesta B. Robot-assisted thoracoscopic lobectomy for early-stage lung cancer. Ann Thorac Surg 2008;85:1880-5; discussion 1885-6.
- Melfi FM, Mussi A. Robotically assisted lobectomy: learning curve and complications. Thorac Surg Clin 2008;18:289-95. [PubMed]
- Veronesi G, Galetta D, Maisonneuve P, et al. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J Thorac Cardiovasc Surg 2010;140:19-25. [PubMed]
- Marulli G, Schiavon M, Perissinotto E, et al. Surgical and neurologic outcomes after robotic thymectomy in 100 consecutive patients with myasthenia gravis. J Thorac Cardiovasc Surg 2013;145:730-5; discussion 735-6. [PubMed]
- Marulli G, Rea F, Melfi F, et al. Robot-aided thoracoscopic thymectomy for early-stage thymoma: a multicenter European study. J Thorac Cardiovasc Surg 2012;144:1125-30. [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [PubMed]
- Whitson BA, Andrade RS, Boettcher A, et al. Video-assisted thoracoscopic surgery is more favorable than thoracotomy for resection of clinical stage I non-small cell lung cancer. Ann Thorac Surg 2007;83:1965-70. [PubMed]
- Demmy TL, Nwogu C. Is video-assisted thoracic surgery lobectomy better? Quality of life considerations. Ann Thorac Surg 2008;85:S719-28. [PubMed]
- Nakata M, Saeki H, Yokoyama N, et al. Pulmonary function after lobectomy: video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg 2000;70:938-41. [PubMed]
- Whitson BA, Groth SS, Duval SJ, et al. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg 2008;86:2008-16; discussion 2016-8.
- Boffa DJ, Allen MS, Grab JD, et al. Data from The Society of Thoracic Surgeons General Thoracic Surgery database: the surgical management of primary lung tumors. J Thorac Cardiovasc Surg 2008;135:247-54. [PubMed]
- Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-6. [PubMed]
- Swanson SJ, Miller DL, McKenna RJ Jr, et al. Comparing robot-assisted thoracic surgical lobectomy with conventional video-assisted thoracic surgical lobectomy and wedge resection: results from a multihospital database (Premier). J Thorac Cardiovasc Surg 2014;147:929-37. [PubMed]
- Ninan M, Dylewski MR. Total port-access robot-assisted pulmonary lobectomy without utility thoracotomy. Eur J Cardiothorac Surg 2010;38:231-2. [PubMed]
- Dylewski MR, Ohaeto AC, Pereira JF. Pulmonary resection using a total endoscopic robotic video-assisted approach. Semin Thorac Cardiovasc Surg 2011;23:36-42. [PubMed]
- Park BJ, Melfi F, Mussi A, et al. Robotic lobectomy for non-small cell lung cancer (NSCLC): long-term oncologic results. J Thorac Cardiovasc Surg 2012;143:383-9. [PubMed]
- Lee BE, Korst RJ, Kletsman E, et al. Transitioning from video-assisted thoracic surgical lobectomy to robotics for lung cancer: are there outcomes advantages? J Thorac Cardiovasc Surg 2014;147:724-9. [PubMed]
- Kumar R, Jog A, Vagvolgyi B, et al. Objective measures for longitudinal assessment of robotic surgery training. J Thorac Cardiovasc Surg 2012;143:528-34. [PubMed]