
Surgery and stereotactic body radiotherapy for early stage non-small cell lung cancer: review of meta-analyses
Introduction
Lobectomy with systematic lymph node dissection remains the accepted standard for good risk patients with stage I non-small cell lung cancer (NSCLC). In patients with early-stage NSCLC who are medically compromised but potentially operable, treatment modalities are controversial because stereotactic body radiotherapy (SBRT) or stereotactic ablative radiotherapy (SABR) has been increasingly recognized as a favorable alternative to surgical resection for early-stage NSCLC in those patients (1-3).
Whereas several retrospective and observational studies on potentially operable patients undergoing SBRT suggested that SBRT may be a reasonable alternative to surgical resection, the definition of operability varies or ambiguous between reports (4). Given the ambiguous definition of the operability, it has been a challenge to investigate SBRT versus pulmonary resection in operable patients.
The majority of original studies that have sought to answer this important question are largely retrospective cohort studies (2,5,6) and single institution reports (1,7,8) which analyzed relatively small sample sizes. For this reason, recently published meta-analyses have performed quantitative synthesis of pooled data from these original studies to address this question (9-14). In this review we set out to evaluate the published meta-analyses on this comparison.
Search strategy
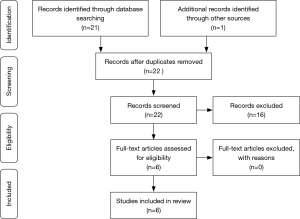
We searched the PubMed (United States National Library of Medicine) to identify original studies published in the English language from inception to July 15th, 2018 using the terms “meta-analysis”, “surgery”, “lung cancer”, and “stereotactic body radiotherapy or stereotactic ablative radiotherapy”. Selection of eligible articles according to preferred reporting items for systematic reviews and meta-analysis (PRISMA) criteria is summarized as workflow in Figure 1. The abstracts of meta-analyses comparing between surgery and SBRT for stages I and II (early stage) NSCLC were and screened for quality, methodology, and description and then selected for full-text review.
Description of published data
In our search for meta-analyses that compare surgery and SBRT for early stage NSCLC, 22 abstracts and 6 full-text papers were evaluated. As a result, six meta-analysis articles were considered to meet eligibility for eligibility and therefore selected for review (9-14).
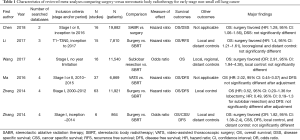
Six meta-analyses comparing surgery and SBRT for early stage NSCLC were comprehensively evaluated in detail (Table 1). Four meta-analyses were published in China, one meta-analysis published in China collaborated with Michigan group), the other published in Netherlands and Canada, between 2014 and 2018. They searched 2 to 4 databases and analyzed a median of 16 original studies, ranging from 6 to 63. The total number of patients in a meta-analysis ranged from 864 to 19,882 patients with a median of 9,675. Three meta-analyses analyzed only original studies on surgically resected stage I NSCLC, while two meta-analyses analyzed patients with surgically resected stages I and II NSCLC. Four of these meta-analyses significantly favored surgery over SBRT in terms of the outcome of overall survival in conclusion. Four meta-analyses examined publication bias and none of them reported a significant publication bias.
Full table
Two meta-analyses pooled data on surgical patients from original studies that contained only surgical patients, while pooled data on SBRT patients from another original studies that contained only SBRT patients. In other words, in the two meta-analyses, data on surgical patients and those on SBRT patients were extracted from two different sets of original studies. On the other hand, the other four meta-analyses analyzed only original studies, each of which contained data on both surgical patients and SBRT patients in their study.
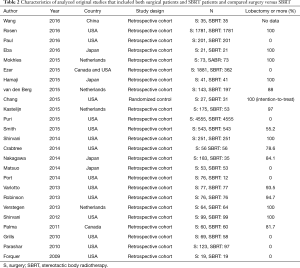
Twenty-five original studies that were analyzed in the above four meta-analyses were summarized in Table 2 (1-3,5-8,15-32). Most original studies (96%) were retrospective cohort studies and the rate of lobectomy varied from 0 to 100% with a median of 82.9%. Fourteen original studies (56%) were published in USA, 4 (16%) in Japan, 4 (16%) in Netherlands, 3 (12%) in others. The sample size ranged from 38 to 9,110 with a median of 152. Among 24 retrospective cohort studies, 19 studies (79.2%) utilized propensity-score matching, 3 studies (12.5%) utilized multivariable analysis, and 2 studies (8.3%) compared surgery and SBRT with no adjustment for potential confounding factors.
Full table
Discussion
A meta-analysis is a quantitative synthesis of measured outcomes from multiple (prospective or retrospective) original studies, attempting to produce a weighted average of the included outcomes. Meta-analyses in general have several advantages that include increasing the statistical power of the analyses which are common to the individual studies and improving estimates of the size of the effect. On the other hand, they also have disadvantages and limitations in relying only on data from previously published studies. For example, it may be impossible to include all relevant studies, either because some studies are not published or because others do not include the outcome of interest, which could be associated with publication bias or selective outcome reporting bias. What is most important is that meta-analyses may inherit the limitations inherent to the original studies including selection bias, information bias, and study designs other than intention-to-treat.
In this review, six meta-analyses were summarized and discussed. Of interest, the measure of effect was not consistent among the reviewed meta-analyses. If primary outcomes in meta-analyses of original studies are related to time-to-event, special considerations are required. Time-to-event outcomes are supposed to take account of not only whether an event takes place but also when the event occurs, therefore both the event itself and the timing of the event are important. Survival outcome such as overall survival is a typical example of a time-to-event outcome. In (prospective or retrospective) cohort studies, it is most appropriate to analyze time-to-event outcomes using hazard ratios (HRs) because HRs appropriately account for both the number and timing of events, and the time until last follow-up for each patient who has not experienced an event (censored) (33,34). Odds ratio and relative risk, however, account only for the number of events and do not take into account when these events occur. It should be noted that odds ratio and relative risk could be measures of effect for survival outcome if all subjects are followed up for a certain period of observation. Specifically, if odds ratio is used for 5-year overall survival, all subjects should be followed up for more than 5 years. Those measures of effect are occasionally reported as survival outcome in several published meta-analyses although other measures of effect, such as HRs were used in the analyzed original studies. Using those measures of effect will introduce inaccuracies in meta-analyses of original studies whose primary outcomes are time-dependent variables such as overall survival and recurrence free survival.
A majority of the original studies comprising the reviewed meta-analyses were retrospective cohort studies. Prospective studies are typically expected to have fewer biases than retrospective studies, whereas even randomized studies, unless properly designed, may add little evidence to pre-existing dataset except for the erroneous conclusion (24). Study designs, whether prospective or retrospective, are of utmost importance when compare surgery and SBRT. In an attempt to perform a meta-analysis, selection of original studies will determine the quality of the meta-analysis. Especially for surgical patients, the approach (open thoracotomy, video-assisted thoracoscopic surgery, or robotic-assisted thoracoscopic surgery), the extent of resection (lobectomy or more, segmentectomy, or wedge resection), and stages of NSCLC should also be as consistent as possible among the included studies.
In conclusion, our review of meta-analyses suggested that surgery, compared with SBRT, may be associated with more favorable overall survival in patients with early stage NSCLC, whereas the results of those meta-analyses should be interpreted with caution due to the above discussions. However, inclusion of appropriate original studies and use of appropriate measures of effect would be associated with more meaningful and more relevant meta-analyses in the future.
In the future, we should seek for metrics, with surgery and SBRT in our view, to select optimal treatment options in individual patients with early stage NSCLC.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Matsuo Y, Chen F, Hamaji M, et al. Comparison of long-term survival outcomes between stereotactic body radiotherapy and sublobar resection for stage I non-small-cell lung cancer in patients at high risk for lobectomy: A propensity score matching analysis. Eur J Cancer 2014;50:2932-8. [Crossref] [PubMed]
- Verstegen NE, Oosterhuis JW, Palma DA, et al. Stage I-II non-small-cell lung cancer treated using either stereotactic ablative radiotherapy (SABR) or lobectomy by video-assisted thoracoscopic surgery (VATS): outcomes of a propensity score-matched analysis. Ann Oncol 2013;24:1543-8. [Crossref] [PubMed]
- Grills IS, Mangona VS, Welsh R, et al. Outcomes after stereotactic lung radiotherapy 411 or wedge resection for stage I non-small-cell lung cancer. J Clin Oncol 2010;28:928-35. [Crossref] [PubMed]
- Onishi H, Shirato H, Nagata Y, et al. Stereotactic body radiotherapy (SBRT) for operable stage I non-small-cell lung cancer: can SBRT be comparable to surgery? Int J Radiat Oncol Biol Phys 2011;81:1352-8. [Crossref] [PubMed]
- Nakagawa T, Negoro Y, Matsuoka T, et al. Comparison of the outcomes of stereotactic body radiotherapy and surgery in elderly patients with cT1-2N0M0 non-small cell lung cancer. Respir Investig 2014;52:221-6. [Crossref] [PubMed]
- Paul S, Lee PC, Mao J, et al. Long term survival with stereotactic ablative radiotherapy (SABR) versus thoracoscopic sublobar lung resection in elderly people: national population based study with propensity matched comparative analysis. BMJ 2016;354:i3570. [Crossref] [PubMed]
- Hamaji M, Chen F, Matsuo Y, et al. Video-assisted thoracoscopic lobectomy versus stereotactic radiotherapy for stage I lung cancer. Ann Thorac Surg 2015;99:1122-9. [Crossref] [PubMed]
- Port JL, Parashar B, Osakwe N, et al. A propensity-matched analysis of wedge resection and stereotactic body radiotherapy for early stage lung cancer. Ann Thorac Surg 2014;98:1152-9. [Crossref] [PubMed]
- Wang HH, Zhang CZ, Zhang BL, et al. Sublobar resection is associated with improved outcomes over radiotherapy in the management of high-risk elderly patients with Stage I non-small cell lung cancer: a systematic review and meta-analysis. Oncotarget 2017;8:6033-42. [PubMed]
- Ma L, Xiang J. Clinical outcomes of video-assisted thoracic surgery and stereotactic body radiation therapy for early-stage non-small cell lung cancer: A meta-analysis. Thorac Cancer 2016;7:442-51. [Crossref] [PubMed]
- Zheng X, Schipper M, Kidwell K, et al. Survival outcome after stereotactic body radiation therapy and surgery for stage I non-small cell lung cancer: a meta-analysis. Int J Radiat Oncol Biol Phys 2014;90:603-11. [Crossref] [PubMed]
- Zhang B, Zhu F, Ma X, et al. Matched-pair comparisons of stereotactic body radiotherapy (SBRT) versus surgery for the treatment of early stage non-small cell lung cancer: a systematic review and meta-analysis. Radiother Oncol 2014;112:250-5. [Crossref] [PubMed]
- Li M, Yang X, Chen Y, et al. Stereotactic body radiotherapy or stereotactic ablative radiotherapy versus surgery for patients with T1-3N0M0 non-small cell lung cancer: a systematic review and meta-analysis. Onco Targets Ther 2017;10:2885-92. [Crossref] [PubMed]
- Chen H, Laba JM, Boldt RG, et al. Stereotactic Ablative Radiation Therapy Versus Surgery in Early Lung Cancer: A Meta-analysis of Propensity Score Studies. Int J Radiat Oncol Biol Phys 2018;101:186-94. [Crossref] [PubMed]
- Crabtree TD, Puri V, Robinson C, et al. Analysis of first recurrence and survival in patients with stage I non-small cell lung cancer treated with surgical resection or stereotactic radiation therapy. J Thorac Cardiovasc Surg 2014;147:1183-91. [Crossref] [PubMed]
- Varlotto J, Fakiris A, Flickinger J, et al. Matched-pair and propensity score comparisons of outcomes of patients with clinical stage I non-small cell lung cancer treated with resection or stereotactic radiosurgery. Cancer 2013;119:2683-91. [Crossref] [PubMed]
- Robinson CG, DeWees TA, El Naqa IM, et al. Patterns of failure after stereotactic body radiation therapy or lobar resection for clinical stage I non-small-cell lung cancer. J Thorac Oncol 2013;8:192-201. [Crossref] [PubMed]
- Shirvani SM, Jiang J, Chang JY, et al. Comparative effectiveness of 5 treatment strategies for early-stage non-small cell lung cancer in the elderly. Int J Radiat Oncol Biol Phys 2012;84:1060-70. [Crossref] [PubMed]
- Palma D, Visser O, Lagerwaard FJ, et al. Treatment of stage I NSCLC in elderly patients: a population-based matched-pair comparison of stereotactic radiotherapy versus surgery. Radiother Oncol 2011;101:240-4. [Crossref] [PubMed]
- Wang P, Zhang D, Guo XG, et al. A propensity-matched analysis of surgery and stereotactic body radiotherapy for early stage non-small cell lung cancer in the elderly. Medicine (Baltimore) 2016;95:e5723. [Crossref] [PubMed]
- Rosen JE, Salazar MC, Wang Z, et al. Lobectomy versus stereotactic body radiotherapy in healthy patients with stage I lung cancer. J Thorac Cardiovasc Surg 2016;152:44-54.e9. [Crossref] [PubMed]
- Ezer N, Veluswamy RR, Mhango G, et al. Outcomes after Stereotactic Body Radiotherapy versus Limited Resection in Older Patients with Early-Stage Lung Cancer. J Thorac Oncol 2015;10:1201-6. [Crossref] [PubMed]
- van den Berg LL, Klinkenberg TJ, Groen HJ, et al. Patterns of Recurrence and Survival after Surgery or Stereotactic Radiotherapy for Early Stage NSCLC. J Thorac Oncol 2015;10:826-31. [Crossref] [PubMed]
- Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015;16:630-7. [Crossref] [PubMed]
- Kastelijn EA, El Sharouni SY, Hofman FN, et al. Clinical Outcomes in Early-stage NSCLC Treated with Stereotactic Body Radiotherapy Versus Surgical Resection. Anticancer Res 2015;35:5607-14. [PubMed]
- Forquer JA, Fakiris AJ, McGarry RC, et al. Treatment options for stage I non-small-cell lung carcinoma patients not suitable for lobectomy. Expert Rev Anticancer Ther 2009;9:1443-53. [Crossref] [PubMed]
- Parashar B, Patel P, Monni S, et al. Limited resection followed by intraoperative seed implantation is comparable to stereotactic body radiotherapy for solitary lung cancer. Cancer 2010;116:5047-53. [Crossref] [PubMed]
- Puri V, Crabtree TD, Bell JM, et al. Treatment Outcomes in Stage I Lung Cancer: A Comparison of Surgery and Stereotactic Body Radiation Therapy. J Thorac Oncol 2015;10:1776-84. [Crossref] [PubMed]
- Shirvani SM, Jiang J, Chang JY, et al. Lobectomy, sublobar resection, and stereotactic ablative radiotherapy for early-stage non-small cell lung cancers in the elderly. JAMA Surg 2014;149:1244-53. [Crossref] [PubMed]
- Eba J, Nakamura K, Mizusawa J, et al. Stereotactic body radiotherapy versus lobectomy for operable clinical stage IA lung adenocarcinoma: comparison of survival outcomes in two clinical trials with propensity score analysis (JCOG1313-A). Jpn J Clin Oncol 2016;46:748-53. [Crossref] [PubMed]
- Mokhles S, Verstegen N, Maat AP, et al. Comparison of clinical outcome of stage I non-small cell lung cancer treated surgically or with stereotactic radiotherapy: results from propensity score analysis. Lung Cancer 2015;87:283-9. [Crossref] [PubMed]
- Smith BD, Jiang J, Chang JY, et al. Cost-effectiveness of stereotactic radiation, sublobar resection, and lobectomy for early non-small cell lung cancers in older adults. J Geriatr Oncol 2015;6:324-31. [Crossref] [PubMed]
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815-34. [Crossref] [PubMed]
- Williamson PR. Aggregate data meta-analysis with time-to-event outcomes. Stat Med 2002;21:3337-51. [Crossref] [PubMed]


