
Should minimally invasive lung adenocarcinoma be transferred from stage IA1 to stage 0 in future updates of the TNM staging system?
Introduction
In 2011, the lung adenocarcinoma classification system was revised by the International Association Study of Lung Cancer (IASLC), American Thoracic Society (ATS), and the European Respiratory Society (ERS) to provide a uniform terminology and diagnostic criteria (1). According to the new revisions, lung adenocarcinomas were classified into adenocarcinoma in situ (AIS), minimally invasive adenocarcinoma (MIA), and invasive adenocarcinoma (IADC). Lesions formerly known as bronchioloalveolar (BAC) without an invasive component were redefined as AIS. Adenocarcinoma ≤3 cm with a predominantly lepidic pattern and no more than 5 mm invasive component, were defined as MIA (2). IADC was always presented with heterogeneous histologic patterns and divided into 5 major subtypes including lepidic (LEP), acinar (ACN), papillary (PAP), micropapillary (MIP) and solid (SOL) predominant patterns (3). The classification system has been comprehensively validated for its prognostic predictive value (4-7).
The 8th IASLC TNM classification staging project for lung cancer divided the category T1 into T1a, T1b and T1c by 1-cm increments of tumor sizes, with each 1-centimeter increment separating tumors from a significantly different prognosis (8,9). Cases with AIS were classified as TisN0M0 at stage 0, while MIA cases were classified as T1a (mi) and stage IA. Recent studies have demonstrated that both AIS and MIA have excellent survival rates, and that the recurrence and lymph node metastasis among these patients is rare, and that the 5-year survival rate is nearly 100% (10,11). On the contrary, even after the curative-intent surgery, 10–20% IADC patients at stage I will still relapse (9,12). MIA then, is clearly different from IADC of stage IA1 in tumor biology and outcomes. In this study, we aimed to compare patients categorized according to the 8th IASLC lung cancer staging project, to determine if MIA could be transferred from stage IA1to stage 0 together with AIS. Our results may help to better understand the survival rates of the early stage lung adenocarcinoma patients, and to improve the management of the affected patients.
Methods
Patients cohort
The ethical committee approval [No. KS(P)1807] of this retrospective study was obtained from the institutional review board of Shanghai chest hospital, at Shanghai Jiao Tong University. Patients who underwent surgical resection between January 2009 and March 2015 in Shanghai Chest hospital were retrospectively reviewed. The inclusion criterion were patients classified, according to the 8th IASLC TNM staging project, with pathological diagnosis of AIS, MIA or stage IA1 lung IADC. Patient’s hematoxylin & eosin stained (HE) slides of surgically resected tumor specimen were available for pathologic review. The exclusion criteria were patients who had multiple nodules, tumor sizes larger than 3 cm, and lymph nodes metastasis or history of malignancy. Finally, a total of 1,524 patients were included in this study.
Clinicopathological evaluation
According to the IASLC/ATS/ERS classification (1), AIS was defined as a small size (≤3 cm) lesion with growth restricted to neoplastic cells along preexisting alveolar structures, which had lacked a vascular, stromal or pleural invasion. MIA was histopathologically defined as a small size (≤3 cm), and solitary lesion with a predominantly lepidic pattern. The invasion depth in greatest dimensions in any focus were less than 5mm. IADCs were specimens which had showed that the lepidic, acinar, papillary, micropapillary or solid growth pattern as the predominant component, and/or the invasion component in at least one focus, measuring more than 5 mm in greatest dimension.
Pathologic TNM Staging was based on the 8th edition of the IASLC lung cancer staging manual. The clinicopathologic features including gender, age, tumor size, surgical procedure and survival status were collected from patients’ medical records.
Surveillance protocol
The definition of the disease-free survival (DFS) rate was specified as from the time of surgery to time of first event (recurrence or metastasis) detected. The definition of the overall survival rate (OS) was specified as the length of the survival time after surgery. Patients with no event(s) were censored at the end of the follow-up period. DFS and OS status were obtained from clinical medical records or telephone follow-ups.
The routine preoperative examinations were conducted as described in our previous publications (13,14), including a head and chest CT scan, upper abdomen sonography, pulmonary function testing, heart sonography and any other necessary blood tests. Positron emission tomography (PET) scans were used to help the clinical TNM staging among patients with suspicious hilum or mediastinal lymph node enlargement. The postsurgical surveillance including physical examination, chest CT, neck and upper abdominal ultrasound examination, and whole-body bone scanning and magnetic resonance imaging (MRI). All patients were advised to follow up regularly after surgery. Chest CT scans and ultrasound examinations of the upper abdomen were advised to be performed every 3 months for the first year after surgery and at 6-month intervals thereafter. Whole-body bone scan and cranial MRIs were performed annually. Additional imaging studies were performed if patients had any symptoms which occurred regardless of the follow-up schedule. For patients who did follow-up in their local hospital regularly, telephone follow-ups were conducted to record the survival status.
Statistical methods
χ2 tests were used to compare categorical and continuous variables between AIS/MIA and the IADC stage IA1 group. Cochran-Mantel-Haenszel test was used to estimate the correlation between the different surgical procedure groups and covariates. The log-rank test was used to compare the differences in DFS and OS between different histologic subtype groups for univariable analysis. The value of statistical significance was set to 0.05 (pooled analysis). Statistical analyses were performed using SPSS software (version 19; SAS Institute, Cary, NC, USA) and GraphPad (Prism 5).
Results
A total of 1,524 patients were included in our cohort. According to the IASLC/ATS/ERS classification, there were 412 (27%) AIS, 675 (44%) MIA and 437 (29%) stage IA1 IADC patients. Among them, 435 (29%) patients were males and 1,089 (71%) patients were females. A total of 579 (38%) patients were less than or equal to 50 years old, 875 (57%) patients were between 51 and 70 years old, while 70 (5%) patients were older than 70 years old. Tumor size of the majority of AIS patients was ≤1 cm (350, 85%). There were 60 (14%) patients with tumor size larger than 1 cm but no bigger than 2 cm and 10 (1%) patients with tumor size larger than 2 cm but no bigger than 3 cm. For patients with MIA, tumor size less than or equal to 1 cm also accounted for the majority (482, 72%). There were 185 (27%) patients with tumor size larger than 1 cm but no bigger than 2 cm and 8 (1%) patients with tumor size larger than 2 cm but no bigger than 3 cm. For all patients, more than half of the patients (848, 56%) had received lobectomy, while 676 (44%) patients had received limited resection including 411 cases of wedge resection and 265 cases of segmentectomy. The demographic features of 1,524 patients are summarized in Table 1.
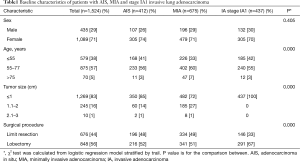
Full table
Survival analysis
At the end of the follow-up, only 8 (0.5%) patients in stage IA1 IADC group experienced a tumor recurrence. The cause of death for the 3 patients in MIA group had no relation to the lung cancer. Three patients in the stage IA1 IADC group died of lung cancer. The median follow-up time for all 1,524 patients in our cohort was 30.6 months.
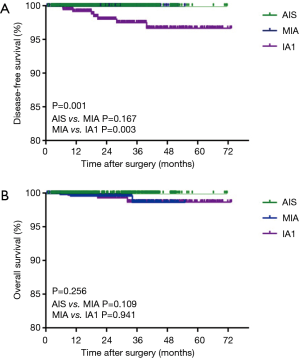
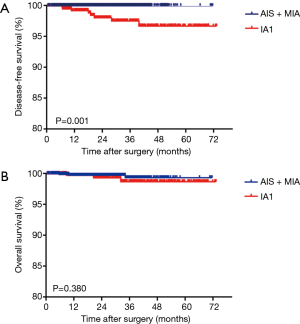
According to the IASLC/ATS/ERS classification, there were no significant DFS and OS [HR, 0.15; 95% confidence interval (CI): 0.02–1.52, P=0.109] differences between patients with AIS and MIA. For patients with stage IA1 IADC, significant worse DFS (HR, 8.27; 95% CI: 2.03–33.64, P=0.003) was seen compared with MIA. However, there was no significant OS (HR, 1.06; 95% CI: 0.21–5.37, P=0.941) difference among these two groups (Figure 1A,B). In order to further explore the possibility of MIA being upgraded to stage 0, together with AIS in the TNM classification system, we combined AIS and MIA as a group and found that there was a significant DFS rate (HR, 20.04; 95% CI: 4.59–87.48; P=0.001) but no OS (HR, 2.14; 95% CI: 0.39–11.78; P=0.380) differences between the IADC group at stage IA1 and the AIS + MIA group (Figure 2A,B).
Risk factors for DFS and OS
In order to identify the risk factors for recurrence or death, multivariable analysis was performed using multivariable Cox models. Multivariable survival analysis which had been adjusted for gender, age, histologic type and surgical strategy, showed that IADC patients of stage IA1 had a no significant DFS (HR, 2.1E5; 95% CI: 0–1.2E100; P=0.912) and OS (HR, 1.76; 95% CI: 0.36–8.73; P=0.489) survival when it had been compared with the AIS + MIA group. Limiting resection (HR, 0.09; 95% CI: 0.02–0.47; P=0.004) was found as a risk factor for the recurrence (Table 2).
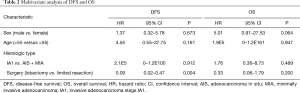
Full table
Discussion
The new IASLC/ATS/ERS lung adenocarcinoma classification system helps to define the various types of lung adenocarcinomas. They have a distinct clinical, radiologic, and histologic characteristic, which could effectively predict the patients’ prognosis, especially among early stage adenocarcinoma patients. In this study, we collected a large single center cohort, to compare the survival among patients with AIS, MIA and stageIA1 IADC. Our results showed that MIA patients have a similar DFS and OS with AIS. However, compared with MIA, there was significant worse DFS for patients with stage IA1 IADC. According to our findings, we suggest that maybe MIA could be moved from stage IA1to stage 0 with AIS and categorized as TmiN0M0 and stage 0.
Evidence showed that the 5-year DFS of MIA patients did not reach 100% (15-19). Behera and colleagues included 19 studies published from 2011 to 2015 to evaluate the prognostic differences between AIS and MIA. Pooled analysis indicated that the 5-year DFS survival rate was 100% for both AIS and MIA, and the 5-year OS rate was 100% for AIS and 98.5% for MIA. Survival analysis found that there were no significant survival differences between the AIS and MIA group. In our cohorts, no patient in AIS and MIA group experienced tumor recurrence, while 3 patients in the MIA group died for reasons beside lung cancer. However, the follow-up time was not enough to determine the prognostic outcomes in patients with AIS and MIA.
According to the data of the IASLC 8th lung cancer TNM staging project (9), the 2-year OS of pathologic stage IA1, IA2 and IA3 was 97%, 94% and 92%, respectively, while the 5-year OS of pathologic stage IA1, IA2 andIA3 was 90%, 85% and 80%, respectively. Distinct survival difference was previously reported between AIS/MIA and stage IA1 patients (20). Consistent with other research, our results specifically demonstrated that patients with MIA had a very close survival rate to those with AIS, while there was a significant DFS difference between AIS/MIA and stage IA1 IADC patients according to univariate analysis. As can be seen, our study posed a critical question of whether MIA should be transferred from IA1 to stage 0 in the next TNM staging edition. This new classification may better guide doctors towards the prognosis and effective management of patients with AIS, MIA and stage IA1 IADC.
Alas, limitations of our study should be declared. First, this is a retrospective study conducted in a single center, and so patient selection bias is inevitable. Second, the follow-up time was not adequate to compare long-term survival for early stage lung cancer. Third, the final pathologic diagnosis may be affected by the experience of different pathologists, even though our hospital is one of the leading thoracic centers with the highest volume in China.
Conclusions
In summary, our results demonstrate that the prognosis of the patients with MIA are as good as those with AIS, but much better than stage IA1 IADC patients. We suggest that a shift of MIA together with AIS, from IA1 to stage 0, may be more reasonable in the future, with an update of the TNM staging system.
Acknowledgements
Funding: This article was supported by Project of Shanghai Science and Technology Committee (18411966100), the Wu Jieping Medical Foundation (320.6750.17525) and the Open Fund of Zhejiang Provincial Top Key Discipline of Pharmacology (YKFJ2-001), National Natural Science Foundation of China (81601995).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The ethical committee approval [No. KS(P)1807] of this retrospective study was obtained from the institutional review board of Shanghai chest hospital, at Shanghai Jiao Tong University.
References
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- He P, Yao G, Guan Y, et al. Diagnosis of lung adenocarcinoma in situ and minimally invasive adenocarcinoma from intraoperative frozen sections: an analysis of 136 cases. J Clin Pathol 2016;69:1076-80. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Tsao MS, Marguet S, Le Teuff G, et al. Subtype Classification of Lung Adenocarcinoma Predicts Benefit From Adjuvant Chemotherapy in Patients Undergoing Complete Resection. J Clin Oncol 2015;33:3439-46. [Crossref] [PubMed]
- Luo J, Huang Q, Wang R, et al. Prognostic and predictive value of the novel classification of lung adenocarcinoma in patients with stage IB. J Cancer Res Clin Oncol 2016;142:2031-40. [Crossref] [PubMed]
- Russell PA, Wainer Z, Wright GM, et al. Does lung adenocarcinoma subtype predict patient survival?: A clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol 2011;6:1496-504. [Crossref] [PubMed]
- Woo T, Okudela K, Mitsui H, et al. Prognostic value of the IASLC/ATS/ERS classification of lung adenocarcinoma in stage I disease of Japanese cases. Pathol Int 2012;62:785-91. [Crossref] [PubMed]
- Rami-Porta R, Bolejack V, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015;10:990-1003.
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Yoshizawa A, Motoi N, Riely GJ, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol 2011;24:653-64. [Crossref] [PubMed]
- Ito M, Miyata Y, Kushitani K, et al. Prediction for prognosis of resected pT1a-1bN0M0 adenocarcinoma based on tumor size and histological status: relationship of TNM and IASLC/ATS/ERS classifications. Lung Cancer 2014;85:270-5. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Akerley W, et al. Non-Small Cell Lung Cancer, Version 6.2015. J Natl Compr Canc Netw 2015;13:515-24. [Crossref] [PubMed]
- Luo J, Wang R, Han B, et al. Analysis of the clinicopathologic characteristics and prognostic of stage I invasive mucinous adenocarcinoma. J Cancer Res Clin Oncol 2016;142:1837-45. [Crossref] [PubMed]
- Luo J, Wang R, Han B, et al. Solid predominant histologic subtype and early recurrence predict poor postrecurrence survival in patients with stage I lung adenocarcinoma. Oncotarget 2017;8:7050-8. [PubMed]
- Tsutani Y, Miyata Y, Mimae T, et al. The prognostic role of pathologic invasive component size, excluding lepidic growth, in stage I lung adenocarcinoma. J Thorac Cardiovasc Surg 2013;146:580-5. [Crossref] [PubMed]
- Behera M, Owonikoko TK, Gal AA, et al. Lung Adenocarcinoma Staging Using the 2011 IASLC/ATS/ERS Classification: A Pooled Analysis of Adenocarcinoma In Situ and Minimally Invasive Adenocarcinoma. Clin Lung Cancer 2016;17:e57-64. [Crossref] [PubMed]
- Nakagiri T, Sawabata N, Morii E, et al. Evaluation of the new IASLC/ATS/ERS proposed classification of adenocarcinoma based on lepidic pattern in patients with pathological stage IA pulmonary adenocarcinoma. Gen Thorac Cardiovasc Surg 2014;62:671-7. [Crossref] [PubMed]
- Takahashi M, Shigematsu Y, Ohta M, et al. Tumor invasiveness as defined by the newly proposed IASLC/ATS/ERS classification has prognostic significance for pathologic stage IA lung adenocarcinoma and can be predicted by radiologic parameters. J Thorac Cardiovasc Surg 2014;147:54-9. [Crossref] [PubMed]
- Kadota K, Villena-Vargas J, Yoshizawa A, et al. Prognostic significance of adenocarcinoma in situ, minimally invasive adenocarcinoma, and nonmucinous lepidic predominant invasive adenocarcinoma of the lung in patients with stage I disease. Am J Surg Pathol 2014;38:448-60. [Crossref] [PubMed]
- Wilshire CL, Louie BE, Horton MP, et al. Comparison of outcomes for patients with lepidic pulmonary adenocarcinoma defined by 2 staging systems: A North American experience. J Thorac Cardiovasc Surg 2016;151:1561-8. [Crossref] [PubMed]


