Mid-term follow-up after suture-less aortic heart valve implantation
Introduction
Aortic stenosis (AS) is the most common valve disease in the adult population (1). It is predicted that the incidence of aortic valve disease will increase due to an ageing population. Aortic valve replacement (AVR) remains the standard treatment among patients with severe AS (2). However, older age and comorbidities significantly increase the operative risk and worsen prognosis (1).
As a result, new strategic options such as transcatheter aortic valve implantation (TAVI) or sutureless AVR (SU-AVR) have emerged in elderly and high-risk patients. TAVI became an accepted, alternative method in selected groups of patients with isolated aortic valve disease. However, these techniques are associated with an increased number of complications such as stroke, arrhythmia, coronary ostium occlusion, paravalvular leak or vascular complications (3-5). It is important, during TAVI, that the native stenosed aortic valve is not removed. This means that the durability of TAVI procedure remains uncertain, especially when available literature on long term outcomes is limited.
Recently, sutureless bioprosthesis became an alternative method to standard AVR or TAVI in high-risk patients. Compared to standard AVR, the advantages of SU-AVR include shorter valve implantation, shorter aortic cross clamp (ACC) and cardiopulmonary bypass (CPB) times and higher valve EOA with beneficial hemodynamics parameters (6-9). In contrast to the TAVI procedure, during SU-AVR, the native stenosed valve is totally removed ensuring good valve fixation on the aortic ring. Good early clinical and hemodynamic outcomes have been reported in several studies (6,8,9). However, although early SU-AVR results are encouraging, there are only a few results reported in the literature on long term follow-up.
Aim
The aim of this study is to present clinical and hemodynamic long term outcomes of 3f Enable sutureless bioprosthesis.
Methods
From June 2005 to July 2008, 27 patients in our institution underwent isolated SU-AVR with the 3f Enable sutureless bioprostheses (ATS Medical, Minneapolis, MN, USA). Patient exclusion criteria were severe deformations of aortic ostium, bicuspid aortic valve, left ventricle ejection fraction <30%, disease of any other heart valve requiring surgery, pathological changes in the ascending aorta (e.g., significant dilatation/aneurysm), systemic connective tissue disease (e.g., Marfan’s syndrome), accompanying coronary heart disease, severe illness of any other organ (e.g., COPD, advanced renal failure, neoplastic disease, stroke with significant neurological deficiency)
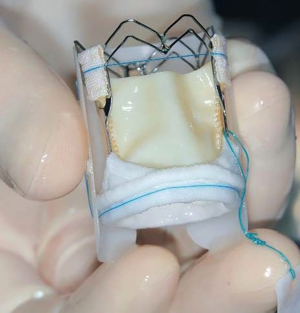
The 3f Enable bioprostheis (Figure 1) is a new-generation sutureless prosthesis consisting of equine pericardium and a self-expanding nitinol frame. The valve is available in 6 sizes: from 19 mm up to 29 mm (in 2 mm steps). Detailed valve description is available in our previous publications (8,9).
Data were collected using a standardized case report and a retrospective analysis was performed. The study was approved by the Bioethics Committee at the Jagiellonian University in Krakow, Poland (No. 122.6120.3235.2014).
Follow-up
TTE were performed before operation, during the first post-operative day, at 3–6, 11–14 months and at 2, 3, 4 and 5 years after the procedure. At each visit, hemodynamic parameters were assessed via TTE by two-dimensional, M-Mode, pulsed-wave and color-flow imaging.
Patients were followed up to 5 years post procedure for SAE and re-operation. One year after the procedure, one patient refused to continue follow-up visit. He was excluded from further analysis. The remaining 25 patients (92.6%) complete 5 years of follow-up
Statistical analysis
Descriptive statistics are presented for baseline demographic and clinical parameters. Continuous variables are presented as mean ± standard deviation (SD) and nominal variables are presented as frequency (%). Statistical analysis was performed using Statistica 10 software (StatSoft Inc., Tulsa, OK, USA).
Results
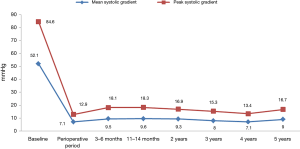
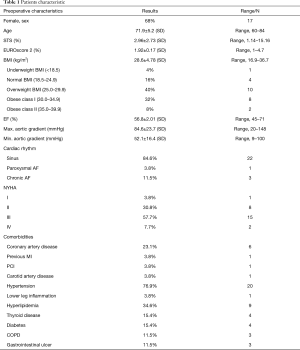
Patient baseline characteristics are presented in Table 1. Mean age was 71.9±5.2 years (range, 60–84 years) and 68% were female. The mean Society of Thoracic Surgeons predicted mortality risk score was 2.96%±2.73% (range, 1.14–15.16%). The mean logistic EuroSCORE II predict operative mortality risk was 1.92%±0.17% (range, 1–4.7%). Echocardiographic results are presented in Table 2. Before AVR, the average peak aortic gradient was 84.6 mmHg and mean aortic gradient was 52.1 mmHg.
Full table
Full table
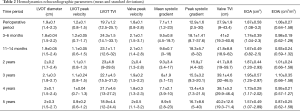
The mean ACC time was 56.0±18.4 min (range, 28–90 min) and CPB was 83.0±22.6 min (range, 40–126 min). After the AVR procedure, the average peak/mean aortic gradients were respectively: 12.9/7.1 mmHg at the intraoperative time; 18.1/9.5 mmHg at 3–6 months; 18.3/9.6 mmHg at 11–14 months; 16.9/9.3 mmHg at 2 years; 15.3/8 mmHg at 3 years; 13.4/7.1 mmHg at 4 years; 16.7/8.9 mmHg at 5 years follow-up. A complete summary of peak and mean trans-prosthetic gradients are present in Figure 2. The EOA and EAOi were respectively: 1.87 cm2 and 1.08 cm2/m2 at the intraoperative time; 1.74 cm2 and 0.98 cm2/m2 at 3–6 months; 1.67 cm2 and 0.93 cm2/m2 at 11–14 months; 1.87 cm2 and 1.01 cm2/m2 at 2 years; 1.95 cm2 and 1.10 cm2/m2 at 3 years; 1.73 cm2 and 0.95 cm2/m2 at 4 years; 1.57 cm2 and 0.87 cm2/m2 at 5 years (Table 2).
Early adverse event rates have been reported. There was one re-operation on post-operative day 4 as a consequence of a significant perivalvular leak caused by small implant displacement (8).
In 5 years of follow-up of the group, there were no deaths, re-operations or other SAEs including thromboembolic complications, endocarditis, pacemaker implantation or valve structure failure were observed. In one patient, after 1 year of follow-up, a small perivalvular leak was detected. However, in further TTE, no increase of the leak was detected, and no re-operation was required. No migration or dislodgement of the prosthesis has occurred during further follow-up.
Discussion
This study is one of the longest follow-up studies of sutureless bioprosthesis. Five years of follow-up demonstrated that the sutureless 3f Enable valve presents an excellent hemodynamic profile with peak and mean gradients of 16.7 and 8.9 mmHg respectively and acceptable clinical outcomes.
AVR remains the gold standard in the treatment in aortic valve disease. Along with the aging population comes a concomitant sharp rise in the incidence of aortic valve disease. However, older age and concomitant comorbidities significantly increase the risk of mortality and morbidity (10,11). Novel sutureless bioprostheses combine the advantages of both techniques: conventional AVR and TAVI. Recently, SU-AVR techniques are proposed as methods of choice in high-risk patients belonging to the “grey zone” between TAVI and conventional surgery (3,12,13). In the literature there are only a few studies that present mid and long term outcomes of SU-AVR.
The hemodynamic parameters of sutureless valves are one of their most important advantages. Sutureless bioprostheses present reduced mean and peak gradients, increased transvalvular flow and acceptable effective orifice area. Our report presents excellent, stable and comparable hemodynamic parameters during 5 years of observation. Compared to short term results from other studies, our results are better or comparable to other SU-AVR studies. Intuity Edwards (8.4 mmHg) (14), Perceval (9.0–12.5 mmHg) (15) and Trilogy bioprostheses were also implanted in the authors’ center (10 mmHg) (16). Similar long term results were report by other centers that implanted the 3f Enable (17). Our results were also comparable with those of conventional tissue valves, including procedures performed in our center (7,18).
The mean EOA values report in our study at each follow-up visit are above the recommended lower limit of 1.2 cm2 and the EOAi mean values are also above the lower limit for mild/not significant (0.85 cm2/m2) prosthesis-patient mismatch (PPM) which is consistent with other study results (17). Short term EOA results are comparable for 3f Enable, Trilogy, Edwards Intuity and slightly lower for Perceval at discharge (1.9 vs. 1.8 vs. 1.7 vs. 1.4 cm2), at 3–6 months (1.7 vs. 1.9 vs. 1.7 vs. 1.5 cm2) and at 1 year after implantation (1.7 vs. 1.9 vs. 1.7 vs. 1.5 cm2) (14,16,19). Sutureless valves present excellent and stable hemodynamic efficacy. Additionally, compared to our center’s experience with conventional stented biological or mechanical heart valves, the sutureless bioprostheses present favorable hemodynamic parameters (18,20). It should be mentioned that, in our study, EOA was calculated using individual echocardiographic parameters that provide a more accurate approach and quality in contrast to studies where the EOA is obtained from previously published studies or in vitro measurements provided by the valve manufactures (17).
The overall rate of serious adverse events observed in our cohort of patients was low. There were no SAEs including endocarditis, thromboembolic complications or pacemaker implantation. Of note, there was no valve structure failure. There was only one re-operation in peri-operative period because of perivalvular leak (3.7%). Of note, similar long term results were obtained among other centers that implanted the Enable valve (17). Also, SU-AVR meta-analysis presents an acceptable rate of SAE, including strokes and re-operations, compared to conventional AVR, supporting the safety of SU-AVR in short term outcomes. It is interesting that there were no significant differences in stroke prevalence between TAVI and SU-AVR. However, these results were published in a small group of patients (3).
An increased rate of pacemaker use in SU-AVR was observed (6.76%) compared to conventional AVR (3.6%) (14,15,21). However, pacemaker use was still lower compared to TAVI where the need for permanent pacemaker implantation was significantly higher and observed in up to one quarter of TAVI patients (3,22). Left bundle branch block was present in 60% of patients after TAVI compare to 7.5% of SU-AVR.
It should be noted that in our study, there were no deaths after 5 years of observation. Meta-analysis showed that early mortality after sutureless bioprostheses was between 2.1% and 3.2% which was lower compared to the mean patients EuroSCORE (21,23). One year mortality rate data showed 2.4% for Perceval (19) and 7.5% for Edwards Intuity (14). In SU-AVR meta-analysis, there was an acceptable rate of mortality from 1.4% to 2.3%, when compared to conventional AVR (14,15). There were no significant differences in in-hospital mortality after SU-AVR (0–2.6%) and TAVI (1.8–5.3%) (5,24). However, score-matched analysis resulted in 144 pairs of intermediate-risk patients with similar baseline characteristics in demonstrating significantly higher in-hospital mortality after TAVI compare to SU-AVR (6.9% vs. 1.4%, P=0.035) (24).
There are only a few studies in the literature that report long term mortality. There are two 5 year observational studies of Perceval that reported higher mortality compare to our results with 71.3% and 85.5% survival after the procedure (25,26). However, our results are difficult to compare because of different patient selection criteria, baseline characteristic and operative risk compare to other studies. Compared to other studies, our cohort of patients was younger, had lower STS score and only the isolated AVR procedure were performed (17,19,27).
There is a perception that sutureless bioprostheses increase the risk of paravalular leaks. In our report, there was one clinically significant paravalular leak 4 days after the procedure that required re-operation and one patient presented with a small PVL without clinical consequences 1 year after the procedure. The highest percentage of PVL has been reported for the Trilogy valve (6.6%). The incidence of PVL for 3f Edwards (2.8%), Perceval (4%) and Intuity (2.3%) have been similar. Of note, the presence of PVL after SU-AVR is significant lower compare to TAVI procedure. The PARTNER trial reported that a trace to mild PVL was observed up to 66% of patients and moderate to severe PVL in 12% (4,28).
Valve migration or dislocation is a severe life-threating complication observed in up to 8% of TAVI procedures (29). This complication may be also observed in the SU-AVR procedure. Although, 3f Enable presented good hemodynamic and clinical outcomes, the valve was withdrawn from the market because of reported valve migration (24). However, along with the increased number of SU-AVR procedure, valve migration is also observed in other types of sutureless bioprostheses (30). In our study, no migration or valve dislocation was observed.
Prolonged ACC and CPB significantly increase the risk of postoperative mortality and morbidity after cardiac surgery (31). The STS database for isolated full sternotomy AVR reports 78 minutes for ACC and 106 minutes for CBP (23). Compare to conventional AVR, SU-AVR implantation time is shorter because of self-expanding bioprostheses and valve fixation in aortic root without suture. Therefore, SU-AVR reduces the duration of operations including ACC and CBP. In our report, mean ACC was 56 min and CBP was 83 min. These results were shorter compare to the STS database, but relatively long compared to results of SU-AVR meta-analysis, where for isolated SU-AVR ACC was 33 min and CBP was 57 min (23). This may be explained by the fact that our study presents the first clinical experience with sutureless bioprostheses, a technique in 2005 which was completely innovative. Of note, along with an increased number of procedures, times were systematically shortened, up to 30 minutes for ACC.
Other advantages of SU-AVR over conventional AVR are the latter requiring longer operations times. Implantation of a sutureless bioprosthesis through a ministernotomy or right minithoracotomy was associated with shorter ACC and CBP and less transfusion of packed red blood cells (5,32-36). According to minimally invasive procedure, sutureless bioprostheses might be a good therapeutic option for patients undergoing multiple concomitant procedures (6,37) including multiple valve-surgery (38). SU-AVR benefits were also observed in high risk cardiac re-operation (37,39), in patients with small aortic annulus (20,40), in porcelain aorta (41) or in calcified homograft (42,43). However, in our center, only isolated full sternotomy AVR procedures were performed. This is common for the first clinical trial involving new types of sutureless valves in new centers (17,23). During the first sutureless valve implantation in the world, investigators preferred isolated AVR procedure with full approach to aorta.
In this study, we demonstrate excellent long term hemodynamic outcomes after SU-AVR using 3f Enable bioprostheses. Five years after the procedure, the peak and mean gradients were 16.7 and 8.9 mmHg, respectively. Unfortunately, due to the lack of long term follow-up, comparisons of different type of sutureless bioprostheses are difficult.
In the authors’ opinion, SU-AVR literature is limited. In recent years, TAVI techniques have become popular in AVR surgery to the detriment of SU-AVR techniques. The advantages and disadvantages of SU-AVR over TAVI and conventional AVR should be demonstrated in large prospective randomized clinical trials which include financial analyses.
Sutureless bioprostheses are a safe and effective treatment for valve stenosis with excellent outcomes and hemodynamic profiles. The advantages of SU-AVR are that this technique can be used in minimally invasive surgery, when concomitant procedures are required and in high risk patients in the “grey zone” between TAVI and conventional surgery.
The limitations of this study that it was a retrospective study, there was a small group of patients and the study lacked a control group. Finally, the study focused mainly on hemodynamics outcomes.
Conclusions
In summary, sutureless bioprostheses are safe and effective treatment for valve stenosis with excellent outcomes and hemodynamic profiles. The peak and mean gradients were 16.7 and 8.9 mmHg respectively after 5 years of follow-up. However further large, randomized studies are needed in order to establish guidelines for SU-AVR in aortic valve disease.
Acknowledgements
None.
Footnote
Conflicts of Interest: B Kapelak and K Bartus were in consultant for ATS Medical, Minneapolis, MN, USA.
Ethical Statement: The study was approved by the Bioethics Committee at the Jagiellonian University in Krakow, Poland (No. 122.6120.3235.2014).
References
- Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J 2003;24:1231-43. [Crossref] [PubMed]
- Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012). The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). G Ital Cardiol (Rome) 2013;14:167-214. [PubMed]
- Muneretto C, Bisleri G, Moggi A, et al. Treating the patients in the 'grey-zone' with aortic valve disease: a comparison among conventional surgery, sutureless valves and transcatheter aortic valve replacement. Interact Cardiovasc Thorac Surg 2015;20:90-5. [Crossref] [PubMed]
- Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597-607. [Crossref] [PubMed]
- Astarci P, Etienne PY, Raucent B, et al. Transcatheter resection of the native aortic valve prior to endovalve implantation - A rational approach to reduce TAVI-induced complications. Ann Cardiothorac Surg 2012;1:224-30. [PubMed]
- Mazine A, Teoh K, Bouhout I, et al. Sutureless aortic valve replacement: a Canadian multicentre study. Can J Cardiol 2015;31:63-8. [Crossref] [PubMed]
- Bartus K, Litwinowicz R, Kusmierczyk M, et al. Primary safety and effectiveness feasibility study after surgical aortic valve replacement with a new generation bioprosthesis: one year outcomes. Kardiol Pol 2018;76:618-24. [PubMed]
- Sadowski J, Kapelak B, Pfitzner R, et al. Sutureless aortic valve bioprothesis '3F/ATS Enable'--4.5 years of a single-centre experience. Kardiol Pol 2009;67:956-63. [PubMed]
- Wendt D, Thielmann M, Buck T, et al. First clinical experience and 1-year follow-up with the sutureless 3F-Enable aortic valve prosthesis. Eur J Cardiothorac Surg 2008;33:542-7. [Crossref] [PubMed]
- Litwinowicz R, Bartus K, Drwila R, et al. In-hospital mortality in cardiac surgery patients after readmission to the intensive care unit: a single-center experience with 10,992 patients. J Cardiothorac Vasc Anesth 2015;29:570-5. [Crossref] [PubMed]
- Litwinowicz R, Bryndza M, Chrapusta A, et al. Hyperbaric oxygen therapy as additional treatment in deep sternal wound infections-a single center's experience. Kardiochir Torakochirurgia Pol 2016;13:198. [Crossref] [PubMed]
- D'Onofrio A, Messina A, Lorusso R, et al. Sutureless aortic valve replacement as an alternative treatment for patients belonging to the "gray zone" between transcatheter aortic valve implantation and conventional surgery: a propensity-matched, multicenter analysis. J Thorac Cardiovasc Surg 2012;144:1010-6. [Crossref] [PubMed]
- Santarpino G, Pfeiffer S, Jessl J, et al. Sutureless replacement versus transcatheter valve implantation in aortic valve stenosis: a propensity-matched analysis of 2 strategies in high-risk patients. J Thorac Cardiovasc Surg 2014;147:561-7. [Crossref] [PubMed]
- Kocher AA, Laufer G, Haverich A, et al. One-year outcomes of the Surgical Treatment of Aortic Stenosis With a Next Generation Surgical Aortic Valve (TRITON) trial: a prospective multicenter study of rapid-deployment aortic valve replacement with the EDWARDS INTUITY Valve System. J Thorac Cardiovasc Surg 2013;145:110-5; discussion 115-6. [Crossref] [PubMed]
- Sian K, Li S, Selvakumar D, et al. Early results of the Sorin(®) Perceval S sutureless valve: systematic review and meta-analysis. J Thorac Dis 2017;9:711-24. [Crossref] [PubMed]
- Breitenbach I, Wimmer-Greinecker G, Bockeria LA, et al. Sutureless aortic valve replacement with the Trilogy Aortic Valve System: multicenter experience. J Thorac Cardiovasc Surg 2010;140:878-84, 884.e1.
- Englberger L, Carrel TP, Doss M, et al. Clinical performance of a sutureless aortic bioprosthesis: five-year results of the 3f Enable long-term follow-up study. J Thorac Cardiovasc Surg 2014;148:1681-7. [Crossref] [PubMed]
- Filip G, Litwinowicz R, Kapelak B, et al. Patient-prosthesis mismatch after minimally invasive aortic valve replacement. Kardiol Pol 2018;76:908-10. [Crossref] [PubMed]
- Folliguet TA, Laborde F, Zannis K, et al. Sutureless perceval aortic valve replacement: results of two European centers. Ann Thorac Surg 2012;93:1483-8. [Crossref] [PubMed]
- Filip G, Bartuś K, Litwinowicz R, et al. Early clinical outcomes of the surgical treatment of patients with aortic stenosis and small aortic annuli. Polish Journal of Thoracic and Cardiovascular Surgery 2013;10:199-203. [Crossref]
- Takagi H, Umemoto T. A Meta-Analysis of Sutureless or Rapid-Deployment Aortic Valve Replacement. Thorac Cardiovasc Surg 2016;64:400-9. [PubMed]
- Biancari F, Barbanti M, Santarpino G, et al. Immediate outcome after sutureless versus transcatheter aortic valve replacement. Heart Vessels 2016;31:427-33. [Crossref] [PubMed]
- Phan K, Tsai YC, Niranjan N, et al. Sutureless aortic valve replacement: a systematic review and meta-analysis. Ann Cardiothorac Surg 2015;4:100-11. [PubMed]
- Concistre G, Miceli A, Chiaramonti F, et al. Delayed dislocation of a sutureless aortic bioprosthesis: the first case. Interact Cardiovasc Thorac Surg 2012;14:892-3. [Crossref] [PubMed]
- Zannis K, Joffre J, Czitrom D, et al. Aortic valve replacement with the perceval S bioprosthesis: single-center experience in 143 patients. J Heart Valve Dis 2014;23:795-802. [PubMed]
- Meuris B, Flameng WJ, Laborde F, et al. Five-year results of the pilot trial of a sutureless valve. J Thorac Cardiovasc Surg 2015;150:84-8. [Crossref] [PubMed]
- Liakopoulos OJ, Gerfer S, Weider S, et al. Direct Comparison of the Edwards Intuity Elite and Sorin Perceval S Rapid Deployment Aortic Valves. Ann Thorac Surg 2018;105:108-14. [Crossref] [PubMed]
- Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187-98. [Crossref] [PubMed]
- Tay EL, Gurvitch R, Wijeysinghe N, et al. Outcome of patients after transcatheter aortic valve embolization. JACC Cardiovasc Interv 2011;4:228-34. [Crossref] [PubMed]
- Amr G, Ghoneim A, Giraldeau G, et al. First case of a sutureless Perceval valve delayed proximal migration. J Thorac Cardiovasc Surg 2017;153:e21-3. [Crossref] [PubMed]
- Ranucci M, Frigiola A, Menicanti L, et al. Aortic cross-clamp time, new prostheses, and outcome in aortic valve replacement. J Heart Valve Dis 2012;21:732-9. [PubMed]
- Dalen M, Biancari F, Rubino AS, et al. Aortic valve replacement through full sternotomy with a stented bioprosthesis versus minimally invasive sternotomy with a sutureless bioprosthesis. Eur J Cardiothorac Surg 2016;49:220-7. [Crossref] [PubMed]
- Aymard T, Kadner A, Walpoth N, et al. Clinical experience with the second-generation 3f Enable sutureless aortic valve prosthesis. J Thorac Cardiovasc Surg 2010;140:313-6. [Crossref] [PubMed]
- Martens S, Ploss A, Sirat S, et al. Sutureless aortic valve replacement with the 3f Enable aortic bioprosthesis. Ann Thorac Surg 2009;87:1914-7. [Crossref] [PubMed]
- Martens S, Sadowski J, Eckstein FS, et al. Clinical experience with the ATS 3f Enable(R) Sutureless Bioprosthesis. Eur J Cardiothorac Surg 2011;40:749-55. [PubMed]
- Gilmanov D, Miceli A, Ferrarini M, et al. Aortic valve replacement through right anterior minithoracotomy: can sutureless technology improve clinical outcomes? Ann Thorac Surg 2014;98:1585-92. [Crossref] [PubMed]
- Concistre G, Santarpino G, Pfeiffer S, et al. Two alternative sutureless strategies for aortic valve replacement: a two-center experience. Innovations (Phila) 2013;8:253-7. [Crossref] [PubMed]
- Mazine A, Minh TH, Bouchard D, et al. Sutureless aortic valve replacement in the presence of a mechanical mitral prosthesis. J Thorac Cardiovasc Surg 2013;146:e27-8. [Crossref] [PubMed]
- Santarpino G, Pfeiffer S, Concistre G, et al. REDO aortic valve replacement: the sutureless approach. J Heart Valve Dis 2013;22:615-20. [PubMed]
- Shrestha M, Maeding I, Hoffler K, et al. Aortic valve replacement in geriatric patients with small aortic roots: are sutureless valves the future? Interact Cardiovasc Thorac Surg 2013;17:778-82; discussion 782. [Crossref] [PubMed]
- Gatti G, Benussi B, Camerini F, et al. Aortic valve replacement within an unexpected porcelain aorta: the sutureless valve option. Interact Cardiovasc Thorac Surg 2014;18:396-8. [Crossref] [PubMed]
- Folliguet TA, Laborde F. Sutureless Perceval aortic valve replacement in aortic homograft. Ann Thorac Surg 2013;96:1866-8. [Crossref] [PubMed]
- Villa E, Messina A, Cirillo M, et al. Perceval sutureless valve in freestyle root: new surgical valve-in-valve therapy. Ann Thorac Surg 2013;96:e155-7. [Crossref] [PubMed]