
The breast cancer patient in the cardioncology unit
Introduction
Over the past 3 decades the breakthroughs of breast cancer (BC) treatment strategies—that included the addition of new generation systemic agents, as well as the use of more advanced and precise radiotherapy techniques—have significantly improved patient’s survival (1). The number of BC survivors is still growing; it has been estimated that nearly three million of them exist at present in the United States, and represent 41% of female cancer survivors. By 2022, BC survivors are expected to become 4.5 million (2,3).
However, to obtain this considerable price has been paid regarding cardiovascular (CV) side effects. Several anti-cancer drugs, commonly used for BC treatment, have the potential to cause CV damage, including acute coronary syndromes, arterial hypertension, arrhythmias, valve impairment, pericarditis, and thrombo-embolic events (4) (Table 1). However, the most feared clinical manifestation of cardiotoxicity is the development of left ventricular dysfunction (LVD) (1,5,6).
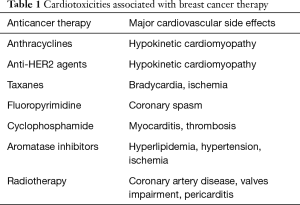
Full table
The incidence of CV injury induced by cancer therapy varies widely, and it depends on the type of drug used, its duration, and underlying patient comorbidities. Recently, in a review of BC survivors, women had a significantly higher risk of death caused by CV disease, that exceeded that of the initial cancer or of recurrent disease (1,7). Indeed, CV disease is the leading cause of death in patients with BC over fifty years of age (1,8). Even when asymptomatic, CV disease not only affects the patient’s cardiology prognosis, but negatively limits therapeutic opportunities when additional therapy for the resumption of cancer or its persistence are needed. As a result, the risk of cardiac adverse events induced by oncologic therapies has become—at present—an important determinant of the BC patient’s survival and quality of life independently of the oncologic prognosis (9).
Cardioncology: a new medical discipline
Since patients previously treated with anticancer therapy have a higher CV risk, the identification and the management of patients with BC at high risk for CV events has become critical in order to reduce morbidity and mortality from CV toxicity due to cancer therapy, which may compromise its effectiveness. A new medical discipline, cardioncology, was born to deal with this need. The neologism was introduced in 1996 (10), and at present, cardioncology is a well-recognized novel medical discipline with the goal being to stimulate a close relationship between cardiologists and oncologists, investigating new strategies, collecting new evidence-based indications, and developing interdisciplinary expertise. Accordingly, a new medical figure has been identified, the cardioncologist, generally a cardiologist, an expert in the management of CV problems in patients with cancer. The cardioncologist’s main aims are to avoid the possibility that cancer therapy could induce cardiac disease—preventing the oncologic patient cured today from becoming the heart patient of tomorrow—and to avoid the possibility that pre-existent cardiac disease be a barrier leading to a reduction of therapeutic opportunities for the patient. In brief, the cardioncologist has to balance cancer care with CV safety, and identify patients who will benefit from a closer CV surveillance or preventive strategies (11).
The cardioncological approach
In the past three years, a number of position statement and guidelines for suggested practices in the field of cardioncology have been developed (12-16). According to these indications, the management of cardiotoxicity refers to four key points: risk stratification, monitoring for early diagnosis, prevention (primary or secondary), and early treatment (Figure 1).
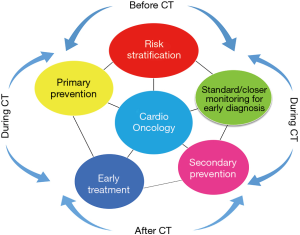
Baseline risk stratification
Breast cancer patients represent the most typical example of cancer patients scheduled for potentially cardiotoxic cancer therapy, being mainly treated with the two classes of drugs—anthracyclines and Her2-monoclonal antibodies—associated with the highest rate of cardiotoxicity. The role of the cardioncologist is to identify patients at low-risk from those at a high-risk. Old patients, patients with a pre-existing CV disease, CV risk factors, prior exposure to chemotherapy (CT) and radiotherapy are patients at increased risk for cardiotoxicity (Table 2) (13). Control and/or correction of hypertension, hypercholesterolemia, diabetes, overweight, and smoking cessation should be the first form of prevention of cardiotoxicity (1,6,13,17,18). The timing and frequency of cardiologic assessment during and after therapy has to be planned, depending on the specific cancer treatment, total cumulative dose, delivery protocol, duration, and the patient’s baseline CV risk. In patients with previous heart disease, the stabilization of the clinical picture, the maximization of cardiac therapy, and a closer and more personalized monitoring scheduling are strongly recommended. Oncologists should also evaluate the CV profile when considering the best anti-cancer pharmacology approach in each patient. Finally, in high-risk patients, a primary prevention—using cardioprotectants and/or CV drugs should be considered (1,6,13,17).
Early detection
In patients at low risk, i.e., without CV risk factors or previous history of cardiac disease, scheduled to receive limited doses of anthracyclines (total cumulative dose ≤240 mg/m2), or limited-dose anthracyclines followed by trastuzumab-based regimens, who represent the largest population of BC patients treated with potentially cardiotoxic therapy, cardiac monitoring is not recommended by the international oncological guidelines, still advising today the identification of cardiotoxicity with the occurrence of symptoms of decompensation (19). Reasons include medicalization, the possibility of causing stress and anxiety, and costs (19,20). Otherwise, the international cardiological guidelines recommend monitoring based on repeated evaluations of left ventricular ejection fraction (LVEF), but are not clear in suggesting when, how often, by what means, nor for how long. However, making a diagnosis of cardiotoxicity based on the onset of symptoms of decompensation or based on evidence of asymptomatic decrease of LVEF is making a very late diagnosis, which precludes any form of effective prevention (21). Moreover, very often at that phase cardiac damage is progressive, and no longer reversible.
A recent prospective study that included a large (n=2,625) unselected population of anthracycline-treated patients (predominantly BC patients; 51%) showed that close monitoring of LVEF after the end of CT enabled almost all cardiotoxicity cases to be identified during the first twelve months of follow-up (22). The study also showed that timely treatment with ace-inhibitors (enalapril) and beta-blockers (carvedilol or bisoprolol) allowed to normalize heart function in most cases (82%). Nonetheless, 11% only of patients who showed normalized LVEF had full recovery, i.e., the same LVEF value as before the start of CT. By contrast, in 71% of patients who had significantly improved and normalized cardiac function, the final LVEF value still remained below the baseline value (Figure 2).
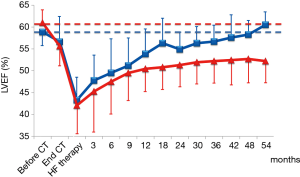
These data confirm that this approach is not very sensitive in identifying cardiotoxicity at an early stage, when it is still potentially reversible, or in predicting a later functional decrease, probably because no changes in LVEF can be observed until a critical extent of myocardial damage occurred, and when compensation mechanisms have been exhausted (9). Furthermore, although a normal LVEF a later loss of cardiac function cannot be excluded.
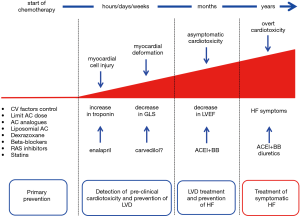
Probably, cardiotoxicity is a continuous phenomenon beginning with myocardial injury, changes in myocardial strain, followed by progressive LVEF decline that may gradually lead to symptomatic heart failure (HF) (Figure 3) (9). We can identify cardiotoxicity at each of these steps, depending on the diagnostic tool we use. At present, we can identify cardiotoxicity at a preclinical phase, very long before HF symptoms onset, long before the evidence of LVEF drop. The majority of data refer to biochemical markers—such as troponins—or cardiac imaging tools (13).
Troponin assessment in breast cancer patients
Troponins are the gold standard biomarkers to detect cardiac damage. In the oncologic setting, several reports demonstrated that troponins are able to detect cardiotoxicity at a pre-clinical stage, in patients treated with different antitumor drugs (23-25) (Table 3) (26-49).
Full table
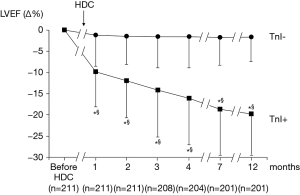
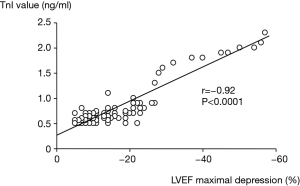
In BC populations, the first study investigating the role of troponins in early cardiotoxicity detection, and in predicting later LVD included 211 patients with poor-prognosis disease, scheduled for high-dose CT (mainly epirubicin; cumulative dose 600 mg/mq) (28). Troponin I (TnI) concentration was assessed before and during three days after each CT. After CT, a different behavior of LVEF in patients showing an increase in the marker (TnI+ group) compared with patients with normal troponin values (TnI– group) has been observed (Figure 4). In the TnI+ group, LVEF significantly reduced after the first month of follow-up, and continued to worse during the follow-up. In the TnI– group, LVEF did not significantly reduce during follow-up (Figure 4). In TnI+ group, TnI maximal value after CT closely correlated with LVEF maximal reduction detected at follow-up (Figure 5).
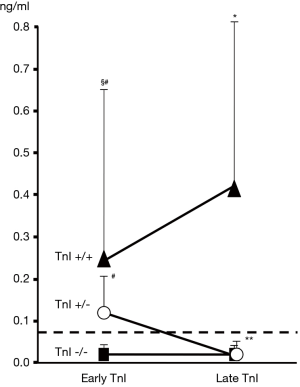
In a following larger study, TnI was measured during three days after CT (early evaluation) and after one month (late evaluation) (31). Three different troponin release patterns were observed: no increase in troponin (TnI−/− patients); increase at early evaluation only (TnI+/− patients); increase at early and late evaluation (TnI+/+ patients) (Figure 6). These three different release patterns were associated with a different cardiological prognosis. A more marked decrease in LVEF and a greater rate of cardiac events was observed in those subjects with troponin elevation, particularly in patients with persistent positivity (Table 4). Given the high negative predictive value identified in this study (99%), TnI identified patients at low-risk, who could be excluded from a close and expensive long-term cardiac surveillance program, reserving a more intensive monitoring to patients at high-risk, i.e., showing an increased positive troponin value, particularly those with persistent positivity (31).
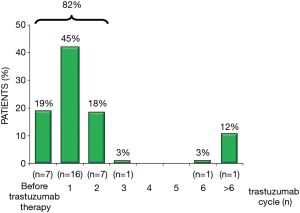
Finally, more recent studies have investigated the potential role of troponins in patients treated with newer cancer agents (Table 3). In a population of 251 BC patients, treated with trastuzumab, troponin increased in 36 cases (14%), most frequently after the first administration (45%; Figure 7) (36). These patients more frequently developed LVD (62% vs. 5%; P<0.001) and were less likely to recover from cardiotoxicity, despite optimized HF therapy. Possibly, Troponin pattern release may allow to differentiate reversible and irreversible cardiac damage in patients treated with a sequential treatment of anthracyclines and trastuzumab. These data have important clinical implications both for oncologists who might decide to resume trastuzumab, and for cardioncologists allowing the identification of patients who need closer monitoring and more aggressive HF therapy (36).
An integrated approach of biomarkers and cardiac imaging
More recently, more sensitive and specific troponin dosing systems have become available thanks to advances in laboratory technology. These new high-sensitivity (HS) dosing systems are able to detect very small levels of troponin which were not identifiable using older troponin dosing methods (48). In the cardioncology field these new tests are very useful in identifying cardiotoxicity, because we often have to deal with very low troponin concentrations, and it is crucial to use high-precision dosing systems (50).
The first study using HS troponin included forty-five subjects with HER-2-overexpressing BC, undergoing anthracyclines treatment, followed by taxanes and trastuzumab. Sawaya et al. assessed global and regional myocardial function by means of tissue Doppler and strain rate imaging in combination with HS troponin I, at baseline and every three months—until fifteen months of follow-up—during anticancer therapy (37). A reduction in longitudinal strain and an increase in HS troponin after the end of anthracycline therapy predicted LVD later development. Notably, the combined evaluation of longitudinal strain and troponin variations showed an increase in specificity (93%) compared to the evaluation of the single parameter (both 73%). In a cohort of patients with BC undergoing the same anticancer schedule, Ky et al. investigated a multimarker assessment (43). All the markers significantly increase from baseline (with the exception of NT-proBNP and galectin-3). Nevertheless, only HS troponin absolute values at the end of anthracycline therapy (before starting taxanes and trastuzumab), and changes in HS troponin I and myeloperoxidase, a marker of oxidative stress, were predictive of later LVD development, even if for the latter marker the significance was less remarkable (43).
Primary prevention: reduction of the direct cardiotoxic effect (Figure 3)
Anthracycline cumulative dose limitation
The risk of doxorubicin-induced HF increases with cumulative dose of anthracycline (1). Current cancer guidelines suggest to limit the maximum cumulative dose of anthracyclines to 450–550 mg/mq, on the basis of results indicating the rapid increase in cardiotoxicity incidence at higher doses (3,6), even if great variability exists in terms of susceptibility to anthracyclines: some patients develop cardiotoxicity at standard doses, while some patients are able to tolerate a total dose two-fold greater than the conventional dose limitation, suggesting that genetic variation might modulate the risk of cardiotoxicity after cancer treatment (6).
Moreover, it’s well known that cardiotoxicity is increased if anthracyclines are associated with trastuzumab (1,3,6). The BCIRG-006 trial compared doxorubicin, cyclophosphamide and docetaxel (ACT), ACT in combination with trastuzumab (ACT-H), and docetaxel, carboplatin in combination with trastuzumab (TCH) regimens for the treatment of HER-2 positive BC (3). Both trastuzumab-containing regimens were more effective than ACT, and similar regarding the oncologic efficacy. Notably, TCH was associated with a significant lower asymptomatic cardiotoxicity, and a lower occurrence of overt HF compared with ACT-H. Thus, minimizing anthracycline exposure, or when possible, the avoidance of anthracycline-based regimens in HER-2+ BC, should be taken into consideration in high-risk patients (1,3,6).
Use of less cardiotoxic anthracycline analogues
Epirubicin demonstrated to have a lower cardiotoxicity in some preclinical and clinical reports (17,19). Epirubicin induced cardiotoxicity occurs after higher doses of doxorubicin, but higher doses must be administered to achieve the same clinical response (90 mg/mq epirubicin =60 mg/mg doxorubicin) (1,9).
Liposomes cannot escape from the vascular space where capillaries have narrow junctions, such as at the heart. Thus, the tendency to accumulate in heart cells is reduced, lowering the risk of cardiotoxicity. Pegylated liposomal doxorubicin showed lower cardiotoxicity compared to standard doxorubicin, and it might be considered in BC subjects at increased risk or need higher doses of anthracycline (3). In a meta-analysis, liposomal doxorubicin showed a lower risk of both asymptomatic, and symptomatic LVD compared to standard doxorubicin (51). Other meta-analyses showed similar results (3,52,53). In addition, a regimen based on non-pegylated liposomal doxorubicin also resulted significantly less cardiotoxic than an epirubicin-based treatment in a small group with non-metastatic BC, without differences in cancer-specific outcomes (54).
Alternatives to trastuzumab for HER-2+ breast cancer
For BC patients with LVD induced by trastuzumab who recovered or partially recovered cardiac function, but who need to continue HER-2 blockade, less toxic, but equally effective alternatives therapeutic options different from trastuzumab should be evaluated. The MARIANNE trial investigated taxanes in combination with trastuzumab (TH) vs. trastuzumab-emtansine (T-DM1) alone or T-DM1 in combination with pertuzumab in patients with advanced HER-2+ BC (55). Both T-DM1-containing regimens were non-inferior to TH regarding progression-free survival and were associated with a lower rate of LVD (22), suggesting that T-DM1 might be a less cardiotoxic choice for those patients needing long-term treatment with trastuzumab and who are at high-risk for cardiotoxicity.
Primary prevention: pharmacologic prevention (Figure 3)
The use of cardioprotectants
The use of cardioprotectants to reduce the cardiotoxic effect of anticancer drugs is of great interest in the cardioncologic context, as an alternative to modifications or limitations/interruptions in cancer treatment (4,50).
Dexrazoxane
Dexrazoxane markedly reduces anthracycline-related cardiotoxicity in adults with different solid tumors and in children with acute lymphoblastic leukemia and Ewing sarcoma (38,56-58). A large amount of evidence shows that patients who received dexrazoxane have a reduced rate of HF as compared to those not receiving this drug. Despite that, dexrazoxane use has not been widely adopted, and it is recommended as a cardioprotectant by the American Society of Clinical Oncology only in subjects with metastatic BC who have already received more than 300 mg/m2 of doxorubicin (58), because of the suspicion never confirmed of an interference with the antitumor effectiveness of anthracyclines (58,59).
The use of cardiovascular agents
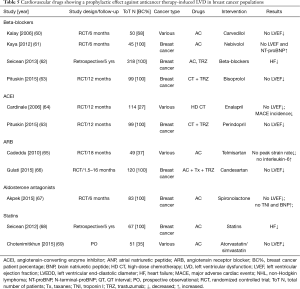
Different classes of drugs—beta-blockers, angiotensin antagonists, statins, and aldosterone antagonists have been reported to be potentially cardioprotective in patients with BC treated with anthracyclines or trastuzumab (Table 5) (60-69).
Full table
Beta-blockers
The cardioprotective effect of carvedilol, a non-cardioselective beta-blocker with antioxidant effects, was shown in a in a small population—mostly with BC—treated with anthracyclines in which the drug was able to prevent LVD (60). In 40 BC patients carvedilol was able to prevent strain abnormalities after anthracycline use, as reported by Elitok et al. (70). In a similar population, the prophylactic use of the drug failed to prevent a LVEF reduction >10% (in all cases, however, the value of LVFE remained within the normal limits), but blunted the troponin increase, and preserved diastolic function (71).
The cardioprotective action of nebivolol, a selective β1 antagonist with nitric oxide-dependent vasodilatory actions, was demonstrated in patients with BC in whom the drug, started seven days before anthracyclines, and administered for six months, prevented the decline of LVEF and NT-proBNP rise. Conversely, in untreated patients, LVEF significantly dropped and the marker increased (61). A retrospective study that included 318 BC patients, the continuation of ongoing beta-blocker therapy during oncology treatment—including anthracyclines, trastuzumab or both—was associated with a lower rate of HF at 5-year follow-up period (62).
Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers
The key role of the renin angiotensin system in the development and progression of cardiotoxicity has been amply demonstrated in experimental models (9,64) In the clinical scenario, the angiotensin II receptor blocker telmisartan, initiated one week before epirubicin in 25 patients with various solid tumors (mostly BC), was able to prevent significant reduction in myocardial deformation variables—as indicated by Tissue Doppler Echocardiography—and an increase in reactive oxygen species or in interleukin-6, exerting its RAS blocking action, and probably also its anti-inflammatory and anti-oxidant properties (65).
Recently, the PRADA study has reported that candesartan—but not metoprolol—administrated with adjuvant CT, with or without trastuzumab, protects against LVEF early decline, as evaluated by cardiac magnetic resonance (66). Conversely, the cardioprotective effectiveness of candesartan has not been confirmed by a following randomized study published in the same year, and involving a very similar population (72).
The Canadian study MANTICORE-101 compared perindopril vs. bisoprolol in the prevention of LVD in patients with HER2+ BC receiving trastuzumab (63). Neither drug has been shown to prevent left ventricular remodeling—defined as an increase in end-diastolic diameters—primary end-point of the study. However, at multivariate analysis, the use of both drugs was associated with a preserved left ventricular function.
Aldosterone antagonists
Spironolactone cardioprotective action has been reported in a recent randomized trial, including 43 BC patients, who started the drug one week before anthracycline-including CT (67). Three weeks after the end of CT—differently from placebo group—they didn’t show significant reductions in LVEF, had a preserved diastolic function, and no increase in troponin I and NT-proBNP.
Statins
Probably, the cardioprotective effect of statins against anthracycline cardiotoxicity depends on their pleiotropic effect, particularly in their antioxidant properties. In a retrospective report, the continuation of ongoing statin use was associated with a noteworthy reduction in HF risk and cardiac mortality during follow-up versus controls (68). More recently, in a prospective observational study from North Carolina, including 51 patients with BC or hematological malignancies, patients already treated with statins for CV prevention, had a lower reduction in LVEF after CT, than those not receiving statins (69).
Secondary prevention
Prevention may be primary, extended to all patients scheduled for potentially cardiotoxic therapies, or secondary, in selected high-risk patients showing preclinical signs of cardiotoxicity as in the form of biomarker increase or strain decrease, with the benefit of limiting prophylactic therapy only to a restricted number of subjects, exposing to possible side effects of the prevention therapy only high-risk patients (Figure 3).
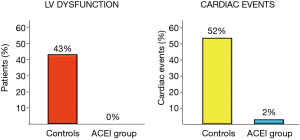
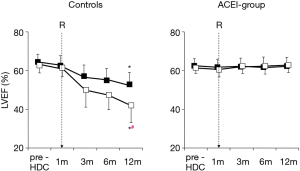
The sole example is a randomized trial including 473 patients with various types of tumors (BC 30%), treated with high-dose CT in which enalapril was evaluated (64). Enalapril was started after the end of CT only in patients showing a troponin rise, titrated as tolerated, and continued for one year. In patients treated with enalapril, no subjects reached the primary end-point i.e., a LVEF reduction of 10 absolute points below the value of 50%, and the incidence of major cardiac events was significantly lower (Figure 8). Notably, in the enalapril-treated group, after a follow-up period of twelve months, LVEF value was equal to baseline value in 88% of cases (both in patients with transient and persistent troponin rise), attesting that enalapril was able to achieve a full preservation of systolic function in this population (Figure 9).
The unique example of preventive therapy in BC patient with evidence of strain parameter reduction is the NCT02177175 trial. The study population has already been treated with anthracyclines and should receive trastuzumab. The inclusion criteria imply a normal LVEF and a reduced longitudinal strain. Patients are randomized to receive or not carvedilol. The main endpoint is the detection of an abnormal LVEF value during the one-year follow-up. The study is still ongoing at the Memorial Sloan Kettering cancer center.
Primary vs. secondary prevention
It has previously been shown that enalapril, started early after evidence of troponin elevation after CT, and continued for twelve months, can prevent the development of LVD and related cardiac events (64). To pick up troponin elevation, however, repeated sampling is necessary, as the marker may increase at different times after therapy infusion, depending on different types of drugs and schedules. Primary prevention, extended to all patients who need to be treated with potentially cardiotoxic anticancer therapies, does not have this limitation (73). The ICOSONE (International CardioOncology Society-one) randomized study was prospectively conducted to compare the effectiveness of two different strategies: to verify whether enalapril, initiated in all patients before CT (Prevention Group) was capable of preventing troponin elevation and subsequent development of LVD, and whether this approach was more effective than enalapril treatment initiated only after evidence of troponin elevation during CT (Troponin-triggered Group). The study enrolled 273 patients (BC 76%) from 21 different oncology centers. Epirubicin and doxorubicin were the most frequently administered anthracyclines [median cumulative dose of 360 (270±360) and 240 (240±240) mg/mq, respectively]. No significant reduction in heart function or very low incidence of CV events was observed in both groups during CT and the twelve-month follow-up. Only three patients (two in the Prevention Group, one in the Troponin-triggered Group; 1.5% vs. 1%; P=NS) developed cardiotoxicity defined as a 10% point reduction of LVEF, below the value of 50%.
Briefly, the main finding of the study was that the two strategies seem equally effective in preventing LVD and adverse cardiac events, confirming the effectiveness of enalapril use in preventing anthracycline-induced LVD, regardless of the strategy chosen.
What strategy should we prefer then? Secondary prevention, guided by a rise in troponin, has the disadvantage that repeated blood sampling is necessary. However, due the very high negative predictive value of the marker highlighted in previous studies (27,28,31,64), this strategy seems justified and cost-effective as it allows the exclusion of low-risk patients, i.e., patients with negative troponin, the majority from long-term surveillance programs by means of costly imaging techniques, with a more favorable cost-benefit ratio, by reducing medicalization, distress, anxiety, and costs (20). In addition, a primary prevention on one hand does not require serial dosing of troponin during CT, on the other hand can be challenging in terms of monitoring during the drug up-titration involving 100% of patients. In addition, extending preventive treatment to all patients treated with CT also exposes those less predisposed to develop cardiotoxicity to possible side-effects (73).
Treatment
Thus far, there are no clear evidence-based recommendations to suggest the best treatment for this category of patients, not least because they have been systematically excluded from the randomized trials that evaluated the current drug therapy for HF. Current recommendations focus principally on the continuation/suspension/renewal of cancer therapy according to the LVEF value of the patient (74). Up until 2010, the few data available that support the use of ace-inhibitors and beta-blockers in patients who developed LVD during or after cancer therapy referred to case reports and old and small retrospective studies (1).
The helpfulness of ace-inhibitors and beta-blockers has been recently evaluated in prospective studies including larger populations of cancer patients (22,75). The results of these studies show that in patients who have developed anthracycline cardiomyopathy therapy with ace-inhibitors and beta-blockers is mandatory, should be started as soon as possible, and increased to the maximum tolerated dosage. A cohort of patients with anthracycline-induced heart disease, an inverse correlation was found between the time elapsed since the end of CT and the beginning of cardiologic therapy (enalapril in association with carvedilol, when possible) and the extent of improvement in LVEF in response to treatment. Actually, 64% of patients treated within two months after the end of CT showed a recovery of LVEF until its normalization; however, in patients treated later this percentage decreased progressively as time passed and no complete recovery was observed in patients treated after six months (Figure 10). Notably, the clinical benefit was most evident in asymptomatic patients (75). These data underline the pivotal importance of an early diagnosis of cardiotoxicity to be able to treat it in a still reversible phase, and suggest, moreover, that a cardiological monitoring focused only on the HF symptoms occurrence, may miss this chance.
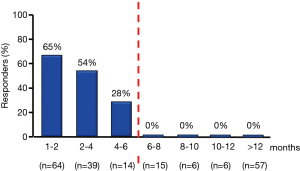
As reported above, a close cardiological surveillance for early diagnosis and a prompt treatment with ACE-inhibitors and beta-blockers have confirmed to be critical for substantial recovery of cardiac function in a broad non selected population treated with anthracycline, has allowed for early detection of 98% of cases of cardiotoxicity during the first twelve months after CT, and led to the normalization of LVEF in 82% of cases (22). However, only 11% of patients had a full recovery (22).
Taken all together, these findings advise that strategies targeted at preventing the development of LVD appear more expedient than treatments aimed at thwarting an already developed dysfunction, which can be now irreversible in most subjects.
Stakeholders in the cardioncology unit
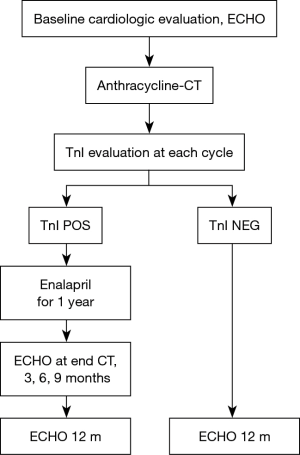
Based on our clinical and scientific experience, we have suggested an approach focused on the identification of high-risk patients for cardiotoxicity by the evaluation of troponin during CT, joined with a prophylactic treatment with enalapril. This approach has been endorsed by the European Society for Medical Oncology (Figure 11) (74). In our routine, in BC patients scheduled for anthracycline-containing CT, we suggest a baseline evaluation, and a troponin evaluation before and soon after every cycle. In case of troponin rise, enalapril is promptly started. The oncologic therapy is not discontinued. The patient is closely monitored during CT and then, during the first year after the completion of CT. Conversely, in patients in whom troponin values remain below the cut-off value, we do not suggest intense cardiac surveillance. This approach is part of an internal procedure, shared also by our oncologist colleagues and it is available on our web site (www.ieo.it). Using this strategy in more than 4,000 patients treated with CT at our institution, we didn’t detect significant drop in LVEF from baseline neither in patients showing nor in those not showing an increase in the marker, and not receiving enalapril, during a twelve-year follow-up.
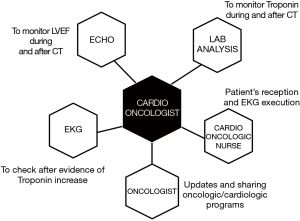
In practical terms, in order to make this kind of approach reliable in our clinical reality, we need the availability, and we have to work closely with colleagues from other disciplines (Figure 12). We need the willingness from our laboratory medicine service for the serial assessment of troponin values during and after the anticancer therapy, the availability of a cardiologist to evaluate EKG and to perform echocardiograms for monitoring LVEF (generally the cardioncologist him/herself), of a cardioncologic nurse to receive the patient at the cardioncology unit, and for performing EKG (especially after troponin rise). Finally, the collaboration with the referral oncologist for updates, and sharing all clinical data for decision making in terms of oncologic therapy, preventive strategies, and a closer monitoring program is of pivotal importance (Figure 12).
Conclusions
Cardioncology is a new interdisciplinary medical area focused on a thorough management of CV complications in patients undergoing cancer treatments. In the last two decades, many aspects have been studied and clarified, though the present evidence-based indications are currently limited. Since the compelling need for skills in this field, cardioncology is a current clinical and research discipline that warrants exploration. This may be fascinating task for both cardiologists and oncologists, particularly for young colleagues, and, as well as, an exciting challenge.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Curigliano G, Cardinale D, Dent S, et al. Cardiotoxicity of anticancer treatments: Epidemiology, detection, and management. CA Cancer J Clin 2016;66:309-25. [Crossref] [PubMed]
- DeSantis CE, Lin CC, Mariotto AB, et al. Cancer Treatment and Survivorship Statistics, 2014. CA Cancer J Clin 2014;64:252-71. [Crossref] [PubMed]
- Caron J, Nohria A. Cardiac toxicity from breast cancer treatment: can we avoid this? Curr Oncol Rep 2018;20:61. [Crossref] [PubMed]
- Colombo A, Cipolla CM, Beggiato M, et al. Cardiac toxicity of anticancer agents. Curr Cardiol Rep 2013;15:362. [Crossref] [PubMed]
- Bloom MW, Hamo CE, Cardinale D, et al. Cancer therapy-related cardiac dysfunction and heart failure: Part 1: definitions, pathophysiology, risk factors, and imaging. Circ Heart Fail 2016;9:e002661. [Crossref] [PubMed]
- Mehta LS, Watson KE, Barac A, et al. Cardiovascular disease and breast cancer: where these entities intersect: a scientific statement from the American Heart Association. Circulation 2018;137:e30-66. [Crossref] [PubMed]
- Bodai BI, Tuso P. Breast cancer survivorship: a comprehensive review of long-term medical issues and lifestyle recommendations. Perm J 2015;19:48-79. [Crossref] [PubMed]
- Pinder MC, Duan Z, Goodwin JS, et al. Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. J Clin Oncol 2007;25:3808-15. [Crossref] [PubMed]
- Cardinale D, Biasillo G, Cipolla CM. Curing cancer, saving the heart: a challenge that cardioncology should not miss. Curr Cardiol Rep 2016;18:51. [Crossref] [PubMed]
- Cardinale D. A new frontier: cardio-oncology. Cardiologia 1996;41:887-91. [PubMed]
- Minotti G. The International Cardioncology Society-ONE trial: not all that glitters is for cardioncologists only. Eur J Cancer 2018;97:27-9. [Crossref] [PubMed]
- Lenihan D. Cardio-oncology: What is the Best Practice we can all strivre for? Int J Cardiol 2017;241:393-4. [Crossref] [PubMed]
- Zamorano JL, Lancellotti P, Rodriguez Muñoz D, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur J Heart Fail 2017;19:9-42. [Crossref] [PubMed]
- Fradley MG, Brown AC, Shields B, et al. Developing a comprehensive cardio-Oncology program at a cancer institute: the Moffit cancer center experience. Oncol Rev 2017;11:340. [Crossref] [PubMed]
- Barros-Gomez S, Herrmann J, Mulvagh SL, et al. Rationale for setting up a cardio-oncology unit: our experience at Mayo Clinic. Cardio-Oncology 2016;2:5. [Crossref]
- Barac A, Murthag G, Carver JR, et al. Cardiovascular health of patients with cancer and cancer survivors. J Am Coll Cardiol 2015;65:2739-46. [Crossref] [PubMed]
- Hamo CE, Bloom MW, Cardinale D, et al. Cancer therapy-related cardiac dysfunction and heart failure: Part 2: prevention, treatment, guidelines, and future directions. Circ Heart Fail 2016;9:e002843. [Crossref] [PubMed]
- Guenancia C, Lefevbvre A, Cardinale D, et al. Obesity as a risk factor for anthracyclines and trastuzumab cardiotoxicity in breast cancer: a systematic review and meta-analysis. J Clin Oncol 2016;34:3157-65. [Crossref] [PubMed]
- Armenian SH, Lacchetti C, Barac A, et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2017;35:893-911. [Crossref] [PubMed]
- Levis BE, Binkley PF, Shapiro CL. Cardiotoxic effects of anthracycline-based therapy: what is the evidence and what are the potential harms? Lancet Oncol 2017;18:e445-56. [Crossref] [PubMed]
- Cardinale D, Cipolla CM. Chemotherapy-induced cardiotoxicity: importance of early detection. Expert Rev Cardiovasc Ther 2016;14:1297-9. [Crossref] [PubMed]
- Cardinale D, Colombo A, Bacchiani G, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015;131:1981-8. [Crossref] [PubMed]
- Christenson ES, James T, Agrawal V, et al. Use of biomarkers for the assessment of chemotherapy-induced cardiac toxicity. Clin Biochem 2015;48:223-35. [Crossref] [PubMed]
- Cardinale D, Biasillo G, Salvatici M, et al. Using biomarkers to predict and to prevent cardiotoxicity of cancer therapy. Expert Rev Mol Diagn 2017;17:245-56. [Crossref] [PubMed]
- Daubert MA, Jeremias A. The utility of troponin measurement to detect myocardial infarction: review of the current findings. Vasc Health Risk Manag 2010;6:691-9. [PubMed]
- Lipshultz SE, Rifai N, Sallan SE, et al. Predictive value of cardiac troponin T in pediatric patients at risk for myocardial injury. Circulation 1997;96:2641-8. [Crossref] [PubMed]
- Cardinale D, Sandri MT, Martinoni A, et al. Left ventricular dysfunction predicted by early troponin I release after high dose chemotherapy. J Am Coll Cardiol 2000;36:517-22. [Crossref] [PubMed]
- Cardinale D, Sandri MT, Martinoni A, et al. Myocardial injury revealed by plasma troponin I in breast cancer treatment with high dose chemotherapy. Ann Oncol 2002;13:710-5. [Crossref] [PubMed]
- Auner HW, Tinchon C, Linkesch W, et al. Prolonged monitoring of troponin T for the detection of anthracycline cardiotoxicity in adults with hematological malignancies. Ann Hematol 2003;82:218-22. [PubMed]
- Sandri MT, Cardinale D, Zorzino L, et al. Minor increases in plasma troponin I predict decreased left ventricular ejection fraction after high-dose chemotherapy. Clin Chem 2003;49:248-52. [Crossref] [PubMed]
- Cardinale D, Sandri MT, Colombo A, et al. Prognostic value of Troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation 2004;109:2749-54. [Crossref] [PubMed]
- Specchia G, Buquicchio C, Pansini N, et al. Monitoring of cardiac function on the basis of treated with anthracyclines. J Lab Clin Med 2005;145:212-20. [Crossref] [PubMed]
- Kilickap S, Barista I, Akgul E, et al. cTnT can be a useful marker for early detection of anthracycline cardiotoxicity. Ann Oncol 2005;16:798-804. [Crossref] [PubMed]
- Lee HS, Son CB, Shin SH, et al. Clinical correlation between brain natriutetic peptide and anthracycline-induced cardiac toxicity. Cancer Res Treat 2008;40:121-6. [Crossref] [PubMed]
- Schmidinger M, Zielinski CC, Vogl UM, et al. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol 2008;26:5204-12. [Crossref] [PubMed]
- Cardinale D, Colombo A, Torrisi R, et al. Trastuzumab-induced cardiotoxicity: clinical and prognostic implications of Troponin I evaluation. J Clin Oncol 2010;28:3910-16. [Crossref] [PubMed]
- Sawaya H, Sebag IA, Plana JC, et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol 2011;107:1375-80. [Crossref] [PubMed]
- Lipshultz SE, Scully RE, Lipsitz SR, et al. Assessment of dexrazoxane as a cardioprotectant in doxorubicin-treated children with high–risk acute lymphoblastic leukaemia: long-term follow-up of a prospective, randomised, multicentre trial. Lancet Oncol 2010;11:950-61. [Crossref] [PubMed]
- Sawaya H, Sebag IA, Plana JC, et al. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes and trastuzumab. Circulation 2012;5:596-603. [PubMed]
- Drafts BC, Twomley KM, D’Agostino R, et al. Low to moderate dose anthracycline-based chemotherapy is associated with early noninvasive imaging evidence of subclinical cardiovascular disease. JACC Cardiovasc Imaging 2013;6:877-85. [Crossref] [PubMed]
- Mornoş C, Petrescu L. Early detection of anthracycline-mediated cardiotoxicity: the value of considering both global longitudinal left ventricular strain and twist. Can J Physiol Pharmacol 2013;91:601-7. [Crossref] [PubMed]
- Mavinkurve-Groothuis AM, Marcus KA, Pourier M, et al. Myocardial 2D strain echocardiography and cardiac biomarkers in children during and shortly after anthracycline therapy for acute lymphoblastic leukaemia: a prospective study. Eur Heart J Cardiovasc Imaging 2013;14:562-9. [Crossref] [PubMed]
- Ky B, Putt M, Sawaya H, et al. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J Am Coll Cardiol 2014;63:809-16. [Crossref] [PubMed]
- Mornoş C, Manolis AJ, Cozma D, et al. The value of left ventricular global longitudinal strain assessed by three-dimensional strain imaging in the early detection of anthracycline-mediated cardiotoxicity. Hellenic J Cardiol 2014;55:235-44. [PubMed]
- Putt M, Hahn VS, Januzzi JL, et al. Longitudinal changes in multiple biomarkers are associated with cardiotoxicity in breast cancer patients treated with Doxorubicin, taxanes, and trastuzumab. Clin Chem 2015;61:1164-72. [Crossref] [PubMed]
- Zardavas D, Suter TM, Van Veldhuisen DJ, et al. Role of troponins I and T and N-terminal prohormone of brain natriuretic peptide in monitoring cardiac safety of patients with early-stage human epidermal growth factor receptor 2-positive breast cancer receiving trastuzumab: a Herceptin adjuvant study cardiac marker substudy. J Clin Oncol 2017;35:878-84. [Crossref] [PubMed]
- Olivieri J, Perna GP, Bocci C, et al. Modern Management of Anthracycline-Induced Cardiotoxicity in Lymphoma Patients: Low Occurrence of Cardiotoxicity with Comprehensive Assessment and Tailored Substitution by Nonpegylated Liposomal Doxorubicin. Oncologist 2017;22:422-31. [Crossref] [PubMed]
- Kitayama H, Kondo T, Sugiyama J, et al. High-sensitive troponin T assay can predict anthracycline- and trastuzumab-induced cardiotoxicity in breast cancer patients. Breast Cancer 2017;24:774-82. [Crossref] [PubMed]
- Shafi A, Siddiqui N, Imtiaz S, et al. Left Ventricular systolic dysfunction predicted by early troponin I release after anthracycline based chemotherapy in breast cancer patients. J Ayub Med Coll Abbottabad 2017;29:266-9. [PubMed]
- Biasillo G, Cipolla CM, Cardinale D. Cardio-oncology: Gaps in Knowledge, Goals, Advances, and Educational Efforts. Curr Oncol Rep 2017;19:55. [Crossref] [PubMed]
- Smith LA, Cornelius VR, Plummer CJ, et al. Cardiotoxicity of anthracycline agents for the treatment of cancer: systematic review and meta-analysis of randomised controlled trials. BMC Cancer 2010;10:337. [Crossref] [PubMed]
- van Dalen EC, van der Pal HJ, Kremer LC. Different dosage schedules for reducing cardiotoxicity in people with cancer receiving anthracycline chemotherapy. Cochrane Database Syst Rev 2016;3:CD005008. [PubMed]
- Rafiyath SM, Rasul M, Lee B, et al. Comparison of safety and toxicity of liposomal doxorubicin vs. conventional anthracyclines: a meta-analysis. Exp Hematol Oncol 2012;1:10. [Crossref] [PubMed]
- Lotrionte M, Palazzoni G, Abbate A, et al. Cardiotoxicity of a non-pegylated liposomal doxorubicin-based regimen versus an epirubicin-based regimen for breast cancer: the LITE (Liposomal doxorubicin-Investigational chemotherapy-Tissue Doppler imaging Evaluation) randomized pilot study. Int J Cardiol 2013;167:1055-7. [Crossref] [PubMed]
- Perez EA, Barrios C, Eiermann W, et al. Trastuzumab emtansine with or without pertuzumab versus trastuzumab plus taxane for human epidermal growth factor receptor 2-positive, advanced breast cancer: primary results from the phase III MARIANNE Study. J Clin Oncol 2017;35:141-8. [Crossref] [PubMed]
- Lipshultz SE, Rifai N, Dalton VM, et al. The effect of dexrazoxane on myocardial injury in doxorubicin-treated children with acute lymphoblastic leukemia. N Engl J Med 2004;351:145-53. [Crossref] [PubMed]
- Sieswerda E, van Dalen EC, Postma A, et al. Medical interventions for treating anthracycline-induced symptomatic and asymptomatic cardiotoxicity during and after treatment for childhood cancer Cochrane Database Syst Rev 2011;9:CD008011. [serial online]. [PubMed]
- Hensley ML, Hagerty KL, Kewalramani T, et al. American Society of Clinical Oncology 2008 clinical practice guideline update: use of chemotherapy and radiation therapy protectants. J Clin Oncol 2009;27:127-45. [Crossref] [PubMed]
- Reichardt P, Tabone MD, Mora J, et al. Risk-benefit of dexrazoxane for preventing anthracycline-related cardiotoxicity: re-evaluating the European labeling. Future Oncol 2018;14:2663-76. [Crossref] [PubMed]
- Kalay N, Basar E, Ozdogru I, et al. Protective effects of carvedilol against anthracycline-induced cardiomyopathy. J Am Coll Cardiol 2006;48:2258-62. [Crossref] [PubMed]
- Kaya MG, Ozkan M, Gunebakmaz O, et al. Protective effects of nebivolol against anthracycline-induced cardiomyopathy: a randomized control study. Int J Cardiol 2013;167:2306-10. [Crossref] [PubMed]
- Seicean S, Seicean A, Alan N, et al. Cardioprotective effect of β-adrenoceptor blockade in patients with breast cancer undergoing chemotherapy: follow-up study of heart failure. Circ Heart Fail 2013;6:420-6. [Crossref] [PubMed]
- Pituskin E, Mackey JR, Koshman S, et al. Multidisciplinary Approach to Novel Therapies in Cardio-Oncology Research (MANTICORE 101-Breast): A Randomized Trial for the Prevention of Trastuzumab-Associated Cardiotoxicity. J Clin Oncol 2017;35:870-7. [Crossref] [PubMed]
- Cardinale D, Colombo A, Sandri MT, et al. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation 2006;114:2474-81. [Crossref] [PubMed]
- Cadeddu C, Piras A, Mantovani G, et al. Protective effects of the angiotensin II receptor blocker telmisartan on epirubicin-induced inflammation, oxidative stress, and early ventricular impairment. Am Heart J 2010;160:487.e1-7. [Crossref] [PubMed]
- Gulati G, Heck SL, Røsjø H, et al. Neurohormonal Blockade and Circulating Cardiovascular Biomarkers During Anthracycline Therapy in Breast Cancer Patients: Results From the PRADA (Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy) Study. J Am Heart Assoc 2017.6. [PubMed]
- Akpek M, Ozdogru I, Sahin O, et al. Protective effects of spironolactone against anthracycline-induced cardiomyopathy. Eur J Heart Fail 2015;17:81-9. [Crossref] [PubMed]
- Seicean S, Seicean A, Plana JC, et al. Effect of statin therapy on the risk for incident heart failure in patients with breast cancer receiving anthracycline chemotherapy: an observational clinical cohort study. J Am Coll Cardiol 2012;60:2384-90. [Crossref] [PubMed]
- Chotenimitkhun R, D’Agostino R Jr, Lawrence JA, et al. Chronic statin administration may attenuate early anthracycline-associated declines in left ventricular ejection function. Can J Cardiol 2015;31:302-7. [Crossref] [PubMed]
- Elitok A, Oz F, Cizgici AY, et al. Effect of carvedilol on silent anthracycline-induced cardiotoxicity assessed by strain imaging: A prospective randomized controlled study with six-month follow-up. Cardiol J 2014;21:509-15. [Crossref] [PubMed]
- Avila MS, Ayub-Ferreira SM, de Barros Wanderley MR Jr, et al. Carvedilol for prevention of chemotherapy-related cardiotoxicity: the CECCY Trial. J Am Coll Cardiol 2018;71:2281-90. [Crossref] [PubMed]
- Boekhout AH, Gietema JA, Milojkovic Kerklaan B, et al. Angiotensin II-receptor inhibition with candesartan to prevent trastuzumab-related cardiotoxic effects in patients with early breast cancer: a randomized clinical trial. JAMA Oncol 2016;2:1030-7. [Crossref] [PubMed]
- Cardinale D, Ciceri F, Latini R, et al. ICOS-ONE Study Investigators. Anthracycline-induced cardiotoxicity: A multicenter randomised trial comparing two strategies for guiding prevention with enalapril: The International CardioOncology Society-one trial. Eur J Cancer 2018;94:126-37. [Crossref] [PubMed]
- Curigliano G, Cardinale D, Suter T, et al. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Annals of Oncology 2012;23:vii155-66. [Crossref] [PubMed]
- Cardinale D, Colombo A, Lamantia G, et al. Anthracycline-induced cardiomyopathy. Clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol 2010;55:213-20. [Crossref] [PubMed]




