
Risk of spinal cord ischemia after thoracic endovascular aortic repair
Introduction
Spinal cord ischemia (SCI) had always been a grievous complication of thoracic endovascular aortic repair (TEVAR). It often results in paraplegia, reversible or permanent. A number of patient and procedure-related factors have been shown in previous studies to be associated with the development of SCI after TEVAR, including extensive exclusion of the aorta by endografts (1,2), perioperative hypotension [mean arterial pressure (MAP) <70 mmHg] (3), coverage of the left subclavian artery (LSA) (4,5), less native aorta present proximal to the celiac artery (6), and previous or concomitant thoracic and abdominal aortic repair (1,7).
To prevent or minimize this complication, widely employed prophylactic measures include LSA revascularization (8), cerebrospinal fluid (CSF) drainage (9) and arterial pressure augmentation (10-13). However, SCI still occurred in 0.8–8.6% of the patients in recent reports (2,4,14,15). The present study aimed to evaluate the prevalence and investigate risk for SCI after TEVAR based on current prophylactic measures instituted to address well-known risk factors.
Methods
Consecutive patients who underwent TEVAR successfully between January 2009 and December 2012 in Guangdong Cardiovascular Institute were prospectively enrolled into a database. Retrospective review of data and patients’ electronic medical records was carried out. Indications for TEVAR included type B aortic dissection (AD), thoracic aortic aneurysm (TAA), thoracic aortic intramural hematoma and thoracic aortic rupture. Patients were excluded if: (I) failure to be evaluated for neurological outcomes after TEVAR; (II) presence of lower limbs dysfunction before TEVAR; (III) stroke immediately after TEVAR; (IV) femoral vascular access complications after TEVAR, resulting in lower limbs dysfunction.
Patients were divided into SCI group and non-SCI group depending on new onset of SCI symptoms or signs after TEVAR. The study was approved by the ethics committee of Guangdong General Hospital and informed consents for endovascular aortic repair were obtained from patients and their first degree relatives.
Definitions
SCI was defined as any new onset of transient or permanent paraplegia or paraparesis after TEVAR, manifested as (I) deficit in motor or sensory function of the lower extremities; or (II) urinary or bowel incontinence. SCI was classified as immediate if onset of neurologic deficit was noted without a period of normal function after TEVAR. Otherwise, SCI was classified as delayed (15). Anemia was defined as the level of hemoglobin <120 g/L in males and <110 g/L in females (16). Post-TEVAR hypotension was defined as MAP <65 mmHg lasting for >20 minutes.
Description of endovascular procedure and peri-procedure treatment
Medications were administrated to control systolic blood pressure <120 mmHg and heart rate <80 bpm before TEVAR. TEVAR was performed in a catheterization room under fluoroscopy and angiography. The following endografts were used: Hercules (MicroPort Medical, China), Talent (Medtronic, USA), Valiant (Medtronic, USA), and Zenith (Cook Incorporated, USA).
To facilitate endograft deployment during TEVAR, brief reversible hypotension was achieved by either intravenous sodium nitroprusside, lasting approximately <5 minutes, or rapid artificial cardiac pacing (RACP) (180 beats/min), lasting approximately <10 seconds as reported (17). Access for TEVAR through femoral artery was achieved by surgical incision in the initial part of the study, and it was changed to percutaneous approach since preclosing technique (18) was introduced in the study center in the later part of the study. Patients underwent general anesthesia if TEVAR was performed with the aid of surgical groin incision or immediately after LSA revascularization. Otherwise, local anesthesia was used during TEVAR.
Prophylactic measures in high-risk patients
Patients were deemed as high risk for SCI if (I) more than one endograft was implanted in the descending aorta, or the distal thoracic aorta was covered (the end of endograft reaching within 3 cm to the origin of celiac artery); (II) the patient had previous aortic repair, either thoracic or abdominal, endovascular or surgical. All high-risk patients received prophylactic measures unless contraindicated. Prophylactic measures included LSA revascularization as indicated, blood pressure augmentation, and CSF pressure control after TEVAR.
Selective LSA revascularization with a carotid-subclavian bypass was performed before TEVAR when the LSA coverage is necessary to establish an adequate landing zone in the presence of a dominant or isolated left vertebral artery, or a patent left internal mammary artery-coronary bypass or a functioning left upper extremity arteriovenous dialysis fistula (5,19). Blood pressure augmentation was achieved by sufficient volume resuscitation and vasoconstrictors when necessary, to keep a target MAP >90 mmHg after TEVAR
CSF pressure control was implemented after TEVAR by lumber puncture and CSF withdrawn. Since that CSF drainage with indwelling catheter had been associated with a high risk of infection in our hospital, we performed lumbar punctures for CSF pressure measurement and CSF withdrawn instead of continuous drainage with indwelling catheter (18). The first lumbar puncture was routinely performed at 6 to 24 hours after TEVAR in high-risk patients, or immediately upon manifestation of SCI symptoms or signs. When the reading of CSF pressure was higher than 15 mmHg, CSF was withdrawn to a target pressure reduction of 30%. If the reading of CSF pressure was greater than 10 mmHg and lower than 15 mmHg, CSF was withdrawn to a target pressure <10 mmHg. No indwelling catheter was kept after the target pressure was met, to reduce the risk of infection. For high-risk patients, additional lumbar puncture for CSF pressure control was performed only when the reading of CSF pressure on the previous day was >10 mmHg. For patients who developed SCI, lumbar puncture and CSF withdrawn was performed once daily until there was neither deterioration nor improvement in symptoms, and the reading of CSF pressure on the previous day was <10 mmHg.
Statistical analysis
Normal distribution of the data was assessed with Shapiro-Wilk Test. All the continuous variables were skewed distributed.
Medians (interquartile range) were used to describe continuous variables; intergroup differences were evaluated by nonparametric Mann-Whitney U test. Categorical variables were presented as frequencies. Percentages were compared by Fisher exact test. For stepwise multivariate logistic regression, variables were fitted from the variables found to have marginal associations with SCI on univariate testing (P<0.10). Odds ratios (ORs), 95% confidence intervals (CIs), and probability values are reported. Statistical analyses were performed using SPSS software (Vision 19.0, IBM Inc., USA).
Results
Study population
A total of 664 patients who underwent TEVAR between January 2009 and December 2012 in Guangdong Cardiovascular Institute were screened for the study purpose. Eight patients were excluded because of lower limbs dysfunction before TEVAR and one was excluded because of stroke immediately after TEVAR, to avoid masking symptoms and signs of SCI. Five patients were excluded because of failure to be evaluated for neurological outcome, including two died immediately after TEVAR, one died during the procedure and two developed retrograde type A dissection and were transferred to surgery department for open repair, then lost to follow-up. In total, 14 patients were excluded and 650 patients were enrolled in the study. The median age of the study population was 55.00 (range, 27.00–87.00) years and the majority of them were male (557, 85.69%), hypertensive (529, 81.38%) and with type B AD (549, 84.46%). There was no significant difference between the SCI group and the non-SCI group in terms of age, history of hypertension, prevalence of abdominal aortic aneurysm and coronary artery disease. Prevalence of anemia in the SCI group was significantly higher than that in the non-SCI group (63.6% vs. 26.3%, P=0.015). There was no significant difference in baseline liver, renal and lipid panels between the two groups, while baseline level of hemoglobin in the SCI group was significantly lower (113.00 vs. 128.50, P=0.023) (Table 1). The lowest baseline hemoglobin level in SCI patients before TEVAR was 71 g/L. Due to relative stable hemodynamic status, there was no blood transfusion in patients with anemia before TEVAR.
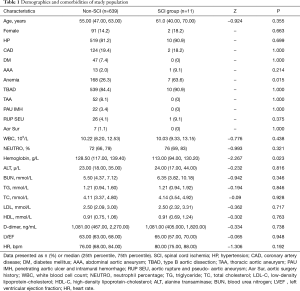
Full table
Treatments and outcomes
Among the 650 patients enrolled in the study, 600 (92.31%) were on β-blockers during hospitalization, 531 (81.69%) underwent local anesthesia, 602 (92.62%) had percutaneous transfemoral access, and 599 (92.15%) had RACP for blood pressure control during TEVAR. Forty (6.15%) patients had endoleak found during TEVAR and 86 (13.23%) had post-TEVAR fever. SCI occurred in 11 (1.69%) patients. Ten (1.54%) patients died during hospital stay and 20 (3.08%) died during the one-year follow-up.
There was no significant difference between the SCI group and the non-SCI group in major medications, number of endograft implanted in descending aorta, presence of immediate endoleak, presence of LSA coverage without revascularization, application of percutaneous femoral access and application of RACP for blood control during TEVAR . More patients in the SCI group underwent general anesthesia (45.5% vs. 17.7%, P=0.033). Notably, patients in the SCI group were significantly more likely to develop post-TEVAR hypotension (27.3% vs. 2.3%, P=0.004).
Ten of the eleven SCI patients underwent elective TEVAR for type B AD and one underwent elective TEVAR for aortic pseudoaneurysm. In all the patients, the development of SCI occurred within the first eight days after TEVAR. SCI developed immediately after TEVAR procedure in six patients while five others had delayed onset. Three of the SCI patients each had an episode of sustained hypotension after TEVAR. The first patient had hypotension on post TEVAR day 2, which was mainly attributed to hemorrhage from surgical procedure for LSA revascularization. SCI was noted on post TEVAR day 3 after the patient was weaned from ventilator. The second patient had an episode of asphyxia associated with profound shock after extubation on post TEVAR day 1. As a result, the patient was sedated and re-intubated. SCI was however noted on post TEVAR day 3 upon awakening from sedation. The third patient developed septic shock following TEVAR. His hypotension was exacerbated by an episode of ventricular tachycardia on post TEVAR day 4, followed by SCI noted on post TEVAR day 5.
At discharge, 4 of the 11 SCI patients had full recovery; another 4 had partial recovery and 3 had no functional improvement. During the one-year follow–up, no later onset of SCI was reported. One SCI patient died of endograft infection during follow-up. The mortality in hospital and mortality at 1 year were not significant different between the SCI and the non-SCI group (0% vs. 1.60%, P=1.000; 9.10% vs. 3.00%, P=0.294, respectively). But length of post-TEVAR stay (13.00 vs. 7.00 days, P=0.000) and length of hospital stay (20.00 vs. 13.00 days, P=0.001) were significantly greater in patients with SCI (Table 2).
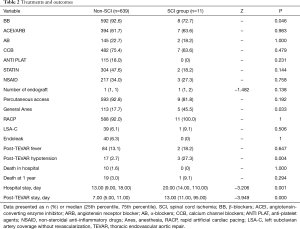
Full table
Risk factors of SCI
Logistic regression analysis was performed with variables of baseline hemoglobin level, general anesthesia and post-TEVAR hypotension. Post-TEVAR hypotension emerged as a significant independent risk factor for SCI (OR, 8.379; 95% CI, 1.833–38.304; P=0.006), while high normal baseline hemoglobin level was a protective factor (OR, 0.969; CI, 0.942–0.998; P=0.037) (Table 3).
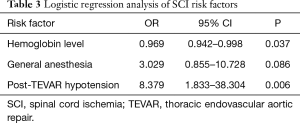
Full table
Discussion
SCI, as one of the major complications after TEVAR, impact on patients’ quality of life seriously and had always been a concern for clinicians. There are multiple established risk factors that are based on patients’ demographic and clinical characteristics, anatomic complexities of the TEVAR procedure, and post repair outcomes. The most frequently reported SCI risk factors include extensive coverage of the aorta by endografts (1,2), perioperative hypotension (MAP <70 mmHg) (3), coverage of the LSA with devices (4), less native aorta present proximal to the celiac artery (6) and previous or concomitant thoracic or abdominal aortic repair (1,7). Preoperative renal insufficiency, older age, occluded hypogastric artery and longer operations may also contribute to the development of SCI (4,20). Based on increasing body of literature and current understanding of SCI, clinicians are using prophylactic measures either to prevent or reduce SCI, including preoperative imaging to identify critical intercostals arteries, prophylactic LSA revascularization (8), CSF drainage to improve spinal cord perfusion (9), intraoperative hypothermia and arterial pressure augmentation (10-13). However, incidence of SCI still ranges between 0.8–8.6% in recent reports (2,4,14,15) and there is a need to further decrease the incidence of this serious complication.
In this study, the incidence of SCI was low as 1.69% in a cohort of patients with type B AD as their major indication for TEVAR (84.46%). However, risk of SCI might differ in different types of aortic pathology. Paraplegia was found in 4.0% of patients with degenerative aneurysm and 0.8% of patients with AD after TEVAR, reported by Dr. Leurs et al., based on combined experience from the EUROSTAR and United Kingdom Thoracic Endograft registries (21). In two recent reports, SCI occurred in 6.6% of patients with thoracic aneurysm (10) and 0.43% of patients with chronic AD (22) respectively. One possible reason for the difference, suggested by Dr. Leurs et al., is that more patients with degenerative aneurysm had multiple endografts implanted, indicating long covered aortic segments (21). Therefore, the low incidence of SCI in the present study was likely partly attributed to the predominant prevalence of type B AD.
We also found a relatively lower mortality rate (1.54%) in the present study, compared with previous reports (23,24). A recent systematic review demonstrated a 30-day mortality of 3.2% after TEVAR for chronic type B AD and 9.8% for acute type B AD (22). Based on the design and purpose of this study, 14 patients were excluded from the patient population, including 2 died immediately after TEVAR, 1 died during TEVAR procedure, 2 had retrograde type A dissection and 1 developed stroke immediately after TEVAR. Undoubtedly, these were high-risk patients and their inclusion in the analysis might have resulted in a higher mortality rate.
Importantly, multivariate analysis demonstrated that post-TEVAR hypotension was a major and independent predictor of SCI. However, owing to the low incidence of SCI and limited study population, the 95% CI for OR of post-TEVAR hypotension was broad (1.83–38.304). Perioperative hypotension had been recognized to be a significant predictor of SCI in studies from around the world (3) and arterial pressure augmentation had been recommended as one of the spinal cord protective measures (1,12,25). Although the majority of patients were hypertensive and prophylactic strategy had emphasized on arterial pressure augmentation, unexpected hypotensive episodes occurred after TEVAR. Hemorrhage associated with surgery or interventional procedure could result in both hypotension and low hemoglobin level, which are directly related to oxygen delivery. In addition, insufficient volume status, overdose of anti-hypertensive agents and acute renal dysfunction, resulting from AD or contrast-induced nephropathy, could lead to unstable hemodynamic status and impaired the spinal cord perfusion. Occasionally, vagal nervous reflection related to vascular procedures and allergic reaction to medications or contrast dyes decrease blood pressure rapidly and profoundly. Periods of missed hypotension such as during patient transfers should also be paid attention to. Our findings highlighted vigilance against hypotension after TEVAR and emphasized awareness on underlying factors that might lead to hypotension, including major hemorrhage, sepsis and complications associated with sedation and intubation. With strict peri-procedure surveillance, most underlying conditions could be avoided or mitigated.
Moreover, the present series revealed that high normal baseline hemoglobin level was a protective factor for SCI. Patients with thoracic AD are often complicated with low level of hemoglobin, which could be the result of blood volume loss in false lumen. Indeed relationship between baseline hemoglobin level and SCI had rarely been studied. The possible mechanism may be due to low level of hemoglobin leading to reduced oxygen carrying capacity and poor oxygen delivery to the spinal cord, exacerbating spinal hypoxia and dysfunction. No wonder, it had been suggested by the European Association for Cardio-Thoracic Surgery that a highly normal serum hemoglobin as well as precise attention to oxygenation will serve to prevent and reverse SCI (26). The importance of this finding is that low level of hemoglobin could be promptly and adequately corrected, especially where other risk factors associated with SCI are difficult to modify. Strategies to improve low level of hemoglobin include nutrition support before elective TEVAR, blood transfusion before emergent TEVAR and prevention of excessive hemorrhage during endovascular procedure as well as adjunctive surgical procedures.
More patients in the SCI group underwent general anesthesia rather than local anesthesia (54.5% vs. 17.7%, P=0.007) in our study, although the difference was not significant on logistic regression. TEVAR were implemented with the aid of either general anesthesia or local anesthesia in different centers. There are no randomized trials comparing anesthetic techniques for TEVAR. In one single-center prospective study that included 400 consecutive TEVAR patients, multivariate analysis revealed that general anesthesia was a risk factor for mortality, compared with regional anesthesia (23). The influence of anesthesia on SCI has not been well described in the literature. General anesthesia usually involves sedatives which influent patient’s hemodynamic status, and associates with more procedure time and ICU stay. When vascular access for TEVAR through femoral artery was achieved via surgical groin incision, general anesthesia was the preferred anesthetic technique. While in recent years, as preclosing technique came into practice in our cardiovascular center, totally percutaneous vascular access with local anesthesia and conscious sedation has become the preferred strategy. Totally percutaneous TEVAR with local anesthesia was reported to be associated with decreased vascular complications, less procedure time and shorter hospital stay (18). Besides TEVAR, other endovascular therapies may benefit from local anesthesia with conscious sedation as well. In transcatheter aortic valve therapy, Dr. Hyman and his colleagues reported that conscious sedation is associated with briefer length of stay and lower in-hospital and 30-day mortality in comparison with general anesthesia (27). Quality improvement outcomes are expected with greater use of local anesthesia and conscious sedation where suitable.
Study limits
Some potential limitations of our study need to be underscored. This is mainly a single institutional retrospective study; hence it might limit the generalization of the conclusions of the study to multi-institutional experience because of difference in patient population and selection criteria. Notwithstanding this potential limitation, we report a current update of a large number of patients in a single high-volume cardiovascular center on important predictors of SCI.
It is important to note that, this cohort excluded cases with post-TEVAR retrograde type A dissection, and cases that died during or immediate after TEVAR. At our cardiovascular center, cardiologists perform endovascular aortic repairs, while open surgical repairs and post-operative care are done by cardiac surgeons. Thus, when TEVAR fails, these patients were transferred to the department of cardiac surgery for open repair. As a result, data of these patients’ follow-up neurological evaluation were not captured in our endovascular repair database, hence their exclusion from the analysis. Their inclusion might have affected overall patient outcomes including SCI.
In addition, our research design for this series did not include the evaluation of aortic anatomical and procedure-related risk factors such as absolute length of aortic exclusion, occlusion of hypogastric artery, quality of TEVAR placing even though these factors had been reported in previous studies to be associated with SCI. Measuring the absolute exclusion of aorta with imaging facilities would provide more precise data for clinical evaluation and statistical analysis. However, in this present series, the number of endograft implanted in the thoracic aorta was used as an estimate of the length of aorta exclusion, since the length of coverage by the proximal endograft was usually around 200 mm. Patients were deemed as high risk if more than one endograft was implanted, which indicated the segment of aorta exclusion >200 mm high-risk patient. This estimation allowed the clinicians to quickly recognize high-risk patients and adopt appropriate prophylactic measures promptly. Because of lack of a control group of high-risk patients without prophylactic measures, we could not tell whether our current prophylactic strategy reduce the risk of SCI. However, the incidence of SCI in the present study is comparable to most recent reports (2,4,15).
Conclusions
The present study confirmed that post-TEVAR hypotension remains a major and independent risk factor for SCI, even with the advent of current practice and prophylactic measures. Clearly, post-TEVAR hypotension should be prevented when possible, identified early and managed aggressively when it occurs. Low level of hemoglobin should always be avoided, as baseline high normal hemoglobin was found to be a protective factor against SCI. Based on our experience, SCI remains an unresolved post TEVAR complication, hence stringent and innovative measures to further decrease SCI are highly recommended.
Acknowledgements
We sincerely thank the reviewers for constructive criticisms and valuable comments, which were of great help in revising the manuscript. Secondly, the manuscript has been systematically improved with better language with the help of Dr. Emmanuel Acheamfour-Akowuah. Our deepest gratitude goes to them.
Funding: This work was supported by the Science and technology Programme of Guangdong Province [2014A020215023]; and the Science and technology Programme of Guangzhou [201300000180, 201508020114 and 201510010282].
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Ethics Committee/Review Board of the Guangdong General Institution (GDREC2015437A), and the requirement for informed patient consent was waived in view of the retrospective nature of the study.
References
- Gravereaux EC, Faries PL, Burks JA, et al. Risk of spinal cord ischemia after endograft repair of thoracic aortic aneurysms. J Vasc Surg 2001;34:997-1003. [Crossref] [PubMed]
- Drinkwater SL, Goebells A, Haydar A, et al. The incidence of spinal cord ischaemia following thoracic and thoracoabdominal aortic endovascular intervention. Eur J Vasc Endovasc Surg 2010;40:729-35. [Crossref] [PubMed]
- Chiesa R, Melissano G, Marrocco-Trischitta MM, et al. Spinal cord ischemia after elective stent-graft repair of the thoracic aorta. J Vasc Surg 2005;42:11-7. [Crossref] [PubMed]
- Buth J, Harris PL, Hobo R, et al. Neurologic complications associated with endovascular repair of thoracic aortic pathology: Incidence and risk factors. a study from the European Collaborators on Stent/Graft Techniques for Aortic Aneurysm Repair (EUROSTAR) registry. J Vasc Surg 2007;46:1103-10; discussion 1110-1. [Crossref] [PubMed]
- Matsumura JS, Rizvi AZ. Left subclavian artery revascularization: Society for Vascular Surgery Practice Guidelines. J Vasc Surg 2010;52:65S-70S. [Crossref] [PubMed]
- Feezor RJ, Martin TD, Hess PJ Jr, et al. Extent of aortic coverage and incidence of spinal cord ischemia after thoracic endovascular aneurysm repair. Ann Thorac Surg 2008;86:1809-14; discussion 1814.
- Kawaharada N, Morishita K, Kurimoto Y, et al. Spinal cord ischemia after elective endovascular stent-graft repair of the thoracic aorta. Eur J Cardiothorac Surg 2007;31:998-1003; discussion 1003. [Crossref] [PubMed]
- Bobadilla JL, Wynn M, Tefera G, et al. Low incidence of paraplegia after thoracic endovascular aneurysm repair with proactive spinal cord protective protocols. J Vasc Surg 2013;57:1537-42. [Crossref] [PubMed]
- Fedorow CA, Moon MC, Mutch WA, et al. Lumbar cerebrospinal fluid drainage for thoracoabdominal aortic surgery: rationale and practical considerations for management. Anesth Analg 2010;111:46-58. [Crossref] [PubMed]
- Cheung AT, Pochettino A, McGarvey ML, et al. Strategies to manage paraplegia risk after endovascular stent repair of descending thoracic aortic aneurysms. Ann Thorac Surg 2005;80:1280-8; discussion 1288-9. [Crossref] [PubMed]
- Weigang E, Hartert M, Siegenthaler MP, et al. Perioperative management to improve neurologic outcome in thoracic or thoracoabdominal aortic stent-grafting. Ann Thorac Surg 2006;82:1679-87. [Crossref] [PubMed]
- Hnath JC, Mehta M, Taggert JB, et al. Strategies to improve spinal cord ischemia in endovascular thoracic aortic repair: Outcomes of a prospective cerebrospinal fluid drainage protocol. J Vasc Surg 2008;48:836-40. [Crossref] [PubMed]
- Boodhwani M, Andelfinger G, Leipsic J, et al. Canadian Cardiovascular Society position statement on the management of thoracic aortic disease. Can J Cardiol 2014;30:577-89. [Crossref] [PubMed]
- Stone DH, Brewster DC, Kwolek CJ, et al. Stent-graft versus open-surgical repair of the thoracic aorta: mid-term results. J Vasc Surg 2006;44:1188-97. [Crossref] [PubMed]
- Khoynezhad A, Donayre CE, Bui H, et al. Risk factors of neurologic deficit after thoracic aortic endografting. Ann Thorac Surg 2007;83:S882-9; discussion S890-2.
- Wan Xuehong LX. Diagnostics: People’s Medical Publishing House, 2013.
- Chen J, Huang W, Luo S, et al. Application of rapid artificial cardiac pacing in thoracic endovascular aortic repair in aged patients. Clin Interv Aging 2014;9:73-8. [PubMed]
- Ni ZH, Luo JF, Huang WH, et al. Totally percutaneous thoracic endovascular aortic repair with the preclosing technique: a case-control study. Chin Med J (Engl) 2011;124:851-5. [PubMed]
- Matsumura JS, Lee WA, Mitchell RS, et al. The Society for Vascular Surgery Practice Guidelines: management of the left subclavian artery with thoracic endovascular aortic repair. J Vasc Surg 2009;50:1155-8. [Crossref] [PubMed]
- Eagleton MJ, Shah S, Petkosevek D, et al. Hypogastric and subclavian artery patency affects onset and recovery of spinal cord ischemia associated with aortic endografting. J Vasc Surg 2014;59:89-94. [Crossref] [PubMed]
- Leurs LJ, Bell R, Degrieck Y, et al. Endovascular treatment of thoracic aortic diseases: combined experience from the EUROSTAR and United Kingdom Thoracic Endograft registries. J Vasc Surg 2004;40:670-9; discussion 679-80. [Crossref] [PubMed]
- Thrumurthy SG, Karthikesalingam A, Patterson BO, et al. A systematic review of mid-term outcomes of thoracic endovascular repair (TEVAR) of chronic type B aortic dissection. Eur J Vasc Endovasc Surg 2011;42:632-47. [Crossref] [PubMed]
- Lee WA, Daniels MJ, Beaver TM, et al. Late outcomes of a single-center experience of 400 consecutive thoracic endovascular aortic repairs. Circulation 2011;123:2938-45. [Crossref] [PubMed]
- Jonker FH, Verhagen HJ, Lin PH, et al. Open surgery versus endovascular repair of ruptured thoracic aortic aneurysms. J Vasc Surg 2011;53:1210-6. [Crossref] [PubMed]
- Ullery BW, Cheung AT, Fairman RM, et al. Risk factors, outcomes, and clinical manifestations of spinal cord ischemia following thoracic endovascular aortic repair. J Vasc Surg 2011;54:677-84. [Crossref] [PubMed]
- Grabenwöger M, Alfonso F, Bachet J, et al. Thoracic Endovascular Aortic Repair (TEVAR) for the treatment of aortic diseases: a position statement from the European Association for Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2012;33:1558-63. [Crossref] [PubMed]
- Hyman MC, Vemulapalli S, Szeto WY, et al. Conscious Sedation Versus General Anesthesia for Transcatheter Aortic Valve Replacement: Insights from the National Cardiovascular Data Registry Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Circulation 2017;136:2132-40. [Crossref] [PubMed]


