A comparison between the efficiency of the Xpert MTB/RIF assay and nested PCR in identifying Mycobacterium tuberculosis during routine clinical practice
Introduction
Tuberculosis (TB) remains a serious public health problem because of its high potential for person-to-person transmission. In Korea, where the prevalence of TB is intermediate, the disease is an important public health issue. In 2012 in Korea, the number of reported TB cases was 49,532, and the estimated annual incidence was 108 per 100,000 people (1). Early diagnosis and rapid introduction of an anti-TB treatment are essential for successful patient outcomes. However, there are shortcomings to the standard diagnostic methods, which include the direct smear for acid-fast bacilli (AFB), which has low sensitivity, and mycobacterial culture, which is slow and usually requires 2-6 weeks to yield a final result (2).
During the last decade, a number of nucleic acid amplification (NAA) methods have been developed for rapid detection and identification of Mycobacterium tuberculosis (MTB) in clinical specimens (3,4). These techniques are attractive because they allow for the direct detection of low MTB genomic copy numbers in specimens. Polymerase chain reaction (PCR) is based on NAA methods and is widely used for the rapid diagnosis of TB.
Our institution currently utilizes two commercial standardized PCR procedures, the Xpert MTB/rifampicin (RIF) assay and MTB nested PCR. MTB nested PCR was developed in an effort to identify the members of the MTB complex. The target is the IS6110 insertion sequence or the mtp40 gene (5,6). The Xpert MTB/RIF assay, using real-time PCR for the TB-specific rpoB gene, is a cartridge-based, automated diagnostic test that can simultaneously identify MTB and resistance to rifampin; it was recently introduced for the rapid diagnosis of TB in Korea. The present study compared the clinical efficiency of the Xpert MTB/RIF assay with that of nested PCR for the detection of MTB among patients with active TB in a newly opened university hospital.
Patients and methods
Study design and specimens
This study was approved by the local institutional review board, with a waiver for obtaining consent from individual patients. We retrospectively examined results from AFB smears and cultures, the Xpert MTB/RIF assay, and MTB nested PCR for 171 patients with suspected TB, in whom those tests were performed at the Hallym University Dongtan Sacred Heart Hospital between February and December 2013. We evaluated the diagnostic accuracy and turnaround time of the Xpert MTB/RIF assay and MTB nested PCR and compared the efficacy of the Xpert MTB/RIF assay with that of the conventional drug susceptibility test (DST) in determining rifampin resistance.
A total of 160 pulmonary and 38 non-pulmonary specimens from 171 suspected TB cases were analyzed in this study. Pulmonary samples included sputum, bronchial washing, and bronchoalveolar lavage fluid, whereas non-pulmonary samples from normally sterile sites consisted of pleural fluid, ascitic fluid, pericardial fluid, joint fluid, cerebrospinal fluid, tissue, or lymph node. If multiple specimens showed positive results from the same patient, only one specimen was used for the analysis.
An initial treatment case of TB was defined as a new patient who had never received treatment for TB or who had taken anti-TB drugs for less than one month. A retreatment case of TB was defined as a patient who was treated after a failure, treated after having previously defaulted, or newly diagnosed with active TB after being previously declared cured or completing treatment (7).
AFB smear and mycobacterial culture
Pulmonary specimens were pretreated with N-acetyl-L-cystein-2% NaOH and centrifugation (3,000 ×g for 20 min at 4 °C). AFB smears were performed using auramine-rhodamine fluorescent staining and confirmed by Ziehl-Neelsen staining. The sediments were inoculated into 3% Ogawa solid media (Asan Pharmaceutical, Seoul, Korea) for eight weeks in 5-10% CO2 incubators, as well as the BD BACTEC MGIT 960 system (Becton, Dickinson, and Company, Sparks, MD, USA), automated liquid culture system, for 6 weeks. Non-pulmonary specimens were cultured without prior pretreatment. Once cultured, MTB was detected using BD MGIT TB identification test, based-on rapid immunochromatography (Becton, Dickinson, and Company).
Drug susceptibility testing (DST)
All positive cultures were tested for DST. It was performed at the Korean Institute of Tuberculosis by the absolute concentration method, considered the gold standard for detection of isoniazid (INH) and RIF resistance, defined as ≥1% bacterial growth in Löwenstein-Jensen medium at concentrations of 0.2 μg/mL for INH and 40.0 μg/mL for RIF, respectively (8).
MTB nested PCR
For nested PCR, the Seeplex® MTB nested ACE Detection assay (Seegene Inc., Seoul, Korea) was performed according to the manufacturer’s instruction. The assay used multi-target (IS6110 and mpb64) PCR, instead of single target PCR for specific detection of MTB. IS6100 is an insertion sequence present in the MTB genome and mpb64 is a conserved sequence present at a single copy in the MTB genome (5,6). The internal control (520 base pairs) a DNA plasmid, was co-amplified with target DNA to identify processed samples containing substances that might interfere with PCR amplification. Amplified PCR products (190 bp) were electrophoresed and visualized on electrophoresis system.
Xpert MTB/RIF assay
The Xpert MTB/RIF assay (Cepheid Inc., Sunnyvale, CA, USA) was performed according to the manufacturer’s instruction. The assay is a fully automated NAA test (by rapid, real-time PCR), was used for the detection of MTB and rifampin resistance. The target was an MTB-specific sequence of the rpoB gene, which was labeled with molecular beacons for mutations within the rifampin resistance determining region (9,10). The testing was carried out on the GeneXpert test device platform, which simplifies molecular testing by fully integrating and automating sample preparation, amplification, and detection. A bacterial buffer was added to the clinical specimens before a defined volume was transferred to a cartridge containing all reagents. The plastic cartridge was then introduced to the GeneXpert device, which provided results in less than two hours.
Statistical analysis
The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the Xpert MTB/RIF assay and nested TB PCR were calculated, and 95% confidence intervals were estimated. The clinical data of the included patients were described with means, medians, and ranges. Continuous variables were compared by Student’s t-test or Mann-Whitney U test (if the variables were not normally distributed), whereas the categorical variables were compared using the chi-square test or Fisher’s exact test. A P value of <0.05 was considered significant. Statistical analyses were performed using dBSTAT software version 4.0 (dBSTAT Inc., Chuncheon, Korea).
Results
Patient characteristics
The Xpert MTB/RIF assay and MTB nested PCR using pulmonary and non-pulmonary clinical specimens were requested for 171 patients with suspicion of TB. The age of these patients was 58.6±17.58, and 104 (60.8%) patients were male. Hypertension, bronchiectasis, diabetes mellitus, and malignancy were the co-morbidities found in 38 (22.2%), 27 (15.8%), 25 (14.6%), and 15 (8.8%) of the patients, respectively. MTB culture-positive pulmonary TB was finally diagnosed in 36 (21.1%). Seven patients (4.1%) were diagnosed with MTB culture-negative pulmonary TB, based on their clinical symptoms and radiographic findings, even though their AFB smears showed positive results. Extrapulmonary TB was diagnosed in six patients (3.5%), TB pleurisy in three, TB meningitis in two, and TB peritonitis in one (Table 1).
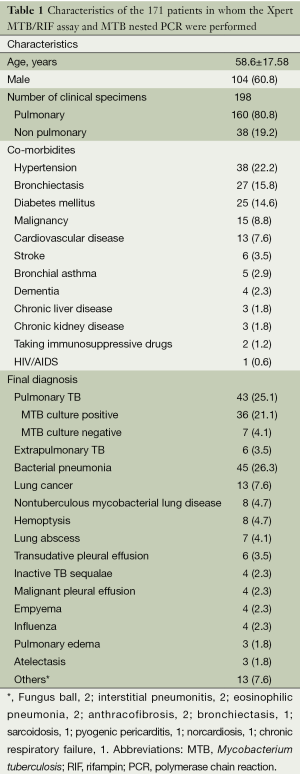
Full table
Comparison of the Xpert MTB/RIF assay and MTB nested PCR
Of 43 pulmonary TB patients, the Xpert MTB/RIF assay and nested PCR yielded positive results in 32 (94.1%) and 26 (78.8%) patients, respectively (P>0.05). Of the cases of MTB culture-positive pulmonary TB, 31 (91.2%) and 25 (75.8%) were found positive by the Xpert MTB/RIF assay and nested PCR, respectively (P>0.05). None of the extrapulmonary TB patients had positive results in any test. Among the patients with false positive results of the Xpert MTB/RIF assay, non-tuberculous mycobacterial (NTM) disease and bacterial pneumonia were identified in each case (2.9%). Of the patients with false positive MTB nested PCR results, bacterial pneumonia was found in three patients (9.1%) and lung cancer, hemoptysis, sarcoidosis, and empyema in one (3.0%) (Table 2).
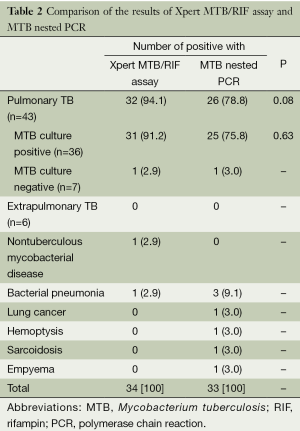
Full table
No differences were observed between the results of the Xpert MTB/RIF assay and MTB nested PCR in patients, according to previous history of treatment for TB (P>0.05). All six patients with extrapulmonary TB corresponded to the initial treatment cases (Table 3). Among the 26 patients available data from conventional DST, only two cases (7.7%) showed resistance to RIF by the Xpert MTB/RIF assay and were found to be multi-drug resistant (MDR) MTB by DST on solid culture (Table 4).
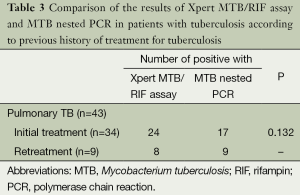
Full table
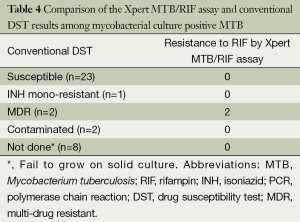
Full table
The median turnaround times from the submission of samples to obtaining results from the laboratory for the Xpert MTB/RIF assay and MTB nested PCR were 0 day (0-4 days) and 4 days (1-11 days), respectively (P<0.001) (Table 5).

Full table
Diagnostic accuracy of the Xpert MTB/RIF assay and MTB nested PCR for mycobacterial culture positive pulmonary TB
The overall sensitivity, specificity, PPV, and NPV of the Xpert MTB/RIF assay and nested PCR for diagnosis of MTB culture positive pulmonary TB were 86.1% (70.49, 95.28) vs. 69.4% (51.89, 83.63), 97.8% (93.63, 99.51) vs. 94.1% (88.65, 97.40), 91.2% (76.30, 98.04) vs. 75.8% (57.74, 88.88), and 96.4% (91.68, 98.79) vs. 92.0% (86.18, 95.95), respectively. No differences were seen among diagnostic accuracies of the Xpert MTB/RIF assay and nested PCR (Table 6).

Full table
Discussion
TB remains a major challenge to public health worldwide, especially in endemic areas, despite global efforts to control the disease. Early identification of MTB is very important, as it can help in the initiation of adequate treatment for patients (11-13). PCR is currently the most promising applicative method in the diagnosis of TB. The technique is based on the amplification of a specific genomic sequence of MTB, which is theoretically highly specific and can be useful in giving a rapid diagnosis. However, variable sensitivity (73-80%) and specificity (80-100%) are obtained with different PCR methods, depending on the area of the genome that is amplified and the techniques used for DNA extraction among different laboratories (14-16). Recently, the Xpert MTB/RIF assay, based on real-time PCR, has been developed in an effort to detect MTB, as well as RIF-resistance TB. Its clinical application, however, is limited in Korea, as it has been introduced relatively recently. Because conventional methods, such as AFB smear and culture, can fail due to the paucibacillary nature of TB and presently used PCR methods often show questionable reliability, an evaluation of newer diagnostic methods for TB is important. Therefore, this study evaluated the efficiency of the Xpert MTB/RIF assay compared to preexisting IS6110- and mtp40-nested PCR to detect MTB in clinical specimens.
In our study, the sensitivity, specificity, PPV, and NPP of the Xpert MTB/RIF assay for diagnosis of MTB culture-positive TB were 86.1%, 97.8%, 91.2%, and 96.4%, respectively, whereas those of nested PCR were 69.4%, 94.1%, 75.8%, and 92.0%, respectively. Out of 43 pulmonary TB patients, no differences were observed between the results of the two tests (P>0.05). Although the sensitivity (86.1%) of the Xpert MTB/RIF assay was higher than that of nested PCR (69.4%), our value was slightly lower than that (90.4%) of a recent study (17). In addition, the turnaround time of the Xpert MTB/RIF assay was shorter than that of the nested PCR, median 0 [0-4] vs. 4 [1-11] days, respectively (P<0.001). These findings suggest that the Xpert MTB/RIF assay is comparable to nested PCR for detection of a case of mycobacterial culture-positive TB among clinical TB suspects; in addition, the shorter turnaround time of the assay may help us to initiate anti-TB therapy promptly.
The number of genomic copies in a specimen can affect a positive PCR result (18). Among 36 pulmonary TB patients with a positive mycobacterial culture, 31 (86.1%) and 25 (69.4%) were found positive with the Xpert MTB/RIF assay and nested PCR, respectively (P>0.05). On the other hand, out of 13 patients, including seven patients with mycobacterial culture-negative pulmonary TB and six patients with extrapulmonary TB, only one patient (7.7%) showed positive results with both tests. These results may come from paucibacillary specimens, such as with TB pleurisy, TB meningitis, and TB peritonitis (19-21). False negative results, however, remain a problem with any test. Among 36 pulmonary MTB culture-positive TB patients, 5 (16.1%) and 11 (30.6%) showed negative results with the Xpert MTB/RIF assay and nested PCR, respectively (P>0.05). A likely reason for the false negative PCR result was the absence of MTB targets (the rpoB gene for the Xpert MTB/RIF assay and the IS6110 or mtp40 gene for nested PCR) in the requested specimens (22,23). Another possible reason was the number of samples submitted for detection of MTB. More than two sets of sputum samples from patients suspected to have TB are normally requested for AFB cultures. However, the cost and labor of PCR often limits the assay to be performed on one specimen because of sampling difficulties, such as bronchial washing, bronchoalveolar lavage fluid, and body fluid from sterile sites. For these reasons, the sensitivity and PPV of the Xpert MTB/RIF assay and nested PCR in our study (86.1% vs. 69.4% and 91.2% vs. 75.8%, respectively) were relatively low compared those in other studies (14-16,21,24).
Compared to the Xpert MTB/RIF assay, nested PCR is time consuming and requires manual labor for sample manipulations, which reduce proteins and enzymes that may inhibit the amplification reactions of specimens. This may cause unintentional errors, such as inappropriate specimen dilution and cross-contamination. In our study, among non-TB patients (n=122), excluding 43 pulmonary TB patents and six extrapulmonary TB patients, two (1.6%) and seven patients (5.7%) were positive with the Xpert MTB/RIF assay and nested PCR, respectively (P>0.05). Indeed, nested PCR required multiple user-dependent steps for manipulations, which may give rise to a cross-contamination. In contrast, the Xpert MTB/RIF assay was automatically and simply performed on the GeneXpert device, thus limiting the carryover contamination.
In Korea, MDR-TB strains cause 2.7-3.9% of new TB and 14.0-27.2% of retreatment cases (8,25). In this study, two cases of RIF resistance by the Xpert MTB/RIF assay were found to be MDR-TB patients, one new and one retreatment case of TB. The WHO recommends that the test should be used as the initial diagnostic method in individuals at risk of having MDR-TB or HIV-associated TB and could be used as a follow-up test to sputum microscopy in areas with a low prevalence of MDR-TB or HIV (1). Likewise, the Xpert MTB/RIF assay is likely to play an additional role in detecting MDR-TB strains, regardless of the past history of therapy for TB, after the increase in notification rate of MDR-TB in Korea (1,24). However, we should consider the false negative aspect of the assay, as it may neither detect MTB nor identify RIF resistance in some MDR-TB strains (21,24).
The limitations of our study include a small sample size, as well as the fact that the submitted specimen was not aliquoted into two portions (one for the Xpert MTB/RIF and the other for nested PCR), because of the small amount of volume and sampling difficulties, such as bronchoscopy and body fluid aspiration. Furthermore, both tests were not performed on the same day, in a blind manner by one technician, and independently. This fact may yield some variations in diagnostic accuracy between the Xpert MTB/RIF and nested PCR. We also must consider the cost-effectiveness of PCR techniques, which limits the number times the test can be repeated.
In conclusion, the Xpert MTB/RIF assay appears to have comparable sensitivity and specificity to the nested PCR technique for the routine diagnosis of mycobacterial culture-positive TB. In addition, the assay provides results in a relatively short period, allowing for faster initiation of anti-TB treatment.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- WHO. Estimates of TB and MDR-TB burden are produced by WHO in consultation with countries. Available online: https://extranet.who.int/sree/Reports?op=Replet&name=%2FWHO_HQ_Reports%2FG2%2FPROD%2FEXT%2FTBCountryProfile&ISO2=KR&LAN=EN&outtype=html
- Centers for Disease Control and Prevention (CDC). Updated guidelines for the use of nucleic acid amplification tests in the diagnosis of tuberculosis. MMWR Morb Mortal Wkly Rep 2009;58:7-10. [PubMed]
- Jouveshomme S, Cambau E, Trystram D, et al. Clinical utility of an amplification test based on ligase chain reaction in pulmonary tuberculosis. Am J Respir Crit Care Med 1998;158:1096-101. [PubMed]
- Piersimoni C, Scarparo C, Piccoli P, et al. Performance assessment of two commercial amplification assays for direct detection of Mycobacterium tuberculosis complex from respiratory and extrapulmonary specimens. J Clin Microbiol 2002;40:4138-42. [PubMed]
- Hasegawa N, Miura T, Ishii K, et al. New simple and rapid test for culture confirmation of Mycobacterium tuberculosis complex: a multicenter study. J Clin Microbiol 2002;40:908-12. [PubMed]
- Magdalena J, Vachee A, Supply P, et al. Identification of a new DNA region specific for members of Mycobacterium tuberculosis complex. J Clin Microbiol 1998;36:937-43. [PubMed]
- WHO (2013). Global tuberculosis report.
- Bai GH, Park YK, Choi YW, et al. Trend of anti-tuberculosis drug resistance in Korea, 1994-2004. Int J Tuberc Lung Dis 2007;11:571-6. [PubMed]
- El-Hajj HH, Marras SA, Tyagi S, et al. Detection of rifampin resistance in Mycobacterium tuberculosis in a single tube with molecular beacons. J Clin Microbiol 2001;39:4131-7. [PubMed]
- Tyagi S, Kramer FR. Molecular beacons in diagnostics. F1000 Med Rep 2012;4:10. [PubMed]
- Pai M, Minion J, Sohn H, et al. Novel and improved technologies for tuberculosis diagnosis: progress and challenges. Clin Chest Med 2009;30:701-16. [PubMed]
- Van Deun A, Martin A, Palomino JC. Diagnosis of drug-resistant tuberculosis: reliability and rapidity of detection. Int J Tuberc Lung Dis 2010;14:131-40. [PubMed]
- Yang XY, Li YP, Mei YW, et al. Time and spatial distribution of multidrug-resistant tuberculosis among Chinese people, 1981-2006: a systematic review. Int J Infect Dis 2010;14:e828-37. [PubMed]
- Aryan E, Makvandi M, Farajzadeh A, et al. Clinical value of IS6110-based loop-mediated isothermal amplification for detection of Mycobacterium tuberculosis complex in respiratory specimens. J Infect 2013;66:487-93. [PubMed]
- Aryan E, Makvandi M, Farajzadeh A, et al. A novel and more sensitive loop-mediated isothermal amplification assay targeting IS6110 for detection of Mycobacterium tuberculosis complex. Microbiol Res 2010;165:211-20. [PubMed]
- Singh A, Kashyap VK. Specific and Rapid Detection of Mycobacterium tuberculosis Complex in Clinical Samples by Polymerase Chain Reaction. Interdiscip Perspect Infect Dis 2012;2012:654694.
- Chang K, Lu W, Wang J, et al. Rapid and effective diagnosis of tuberculosis and rifampicin resistance with Xpert MTB/RIF assay: a meta-analysis. J Infect 2012;64:580-8. [PubMed]
- Helb D, Jones M, Story E, et al. Rapid detection of Mycobacterium tuberculosis and rifampin resistance by use of on-demand, near-patient technology. J Clin Microbiol 48:229-37. [PubMed]
- Morehead RS. Tuberculosis of the pleura. South Med J 1998;91:630-6. [PubMed]
- Bastian I, Rigouts L, Van Deun A, et al. Directly observed treatment, short-course strategy and multidrug-resistant tuberculosis: are any modifications required? Bull World Health Organ 2000;78:238-51. [PubMed]
- Bunsow E, Ruiz-Serrano MJ, López Roa P, et al. Evaluation of GeneXpert MTB/RIF for the detection of Mycobacterium tuberculosis and resistance to rifampin in clinical specimens. J Infect 2014;68:338-43. [PubMed]
- Das S, Paramasivan CN, Lowrie DB, et al. IS6110 restriction fragment length polymorphism typing of clinical isolates of Mycobacterium tuberculosis from patients with pulmonary tuberculosis in Madras, south India. Tuber Lung Dis 1995;76:550-4. [PubMed]
- Kent L, McHugh TD, Billington O, et al. Demonstration of homology between IS6110 of Mycobacterium tuberculosis and DNAs of other Mycobacterium spp.? J Clin Microbiol 1995;33:2290-3. [PubMed]
- Kwak N, Choi SM, Lee J, et al. Diagnostic Accuracy and Turnaround Time of the Xpert MTB/RIF Assay in Routine Clinical Practice. PLoS One 2013;8:e77456. [PubMed]
- Choi JC, Lim SY, Suh GY, et al. Drug resistance rates of Mycobacterium tuberculosis at a private referral center in Korea. J Korean Med Sci 2007;22:677-81. [PubMed]


