Enhanced recovery after surgery (ERAS) programs for esophagectomy
Introduction
A multidisciplinary perioperative treatment protocol aimed at acceleration of patient recovery, enhanced recovery after surgery (ERAS) was first demonstrated in practice by Kehlet and Wilmore in the early 2000s (1). Over the years, various ERAS protocols were developed. Subsequently the ERAS Society released guidelines for ERAS implementation in different surgical disciplines (2-4). Evidence supporting the benefits of ERAS has been growing rapidly in recent years. Randomized clinical trials confirmed that ERAS decreases surgical trauma and the stress response which improved outcomes including reduced length of hospital stay (LOS) and decreased postoperative morbidity. To date, a number of systematic reviews and meta-analyses described those advantages in patients of all ages undergoing colorectal (5,6), bariatric (7), orthopedic (8) or gynecologic (9) surgery, where ERAS protocols can be recognized as successfully implemented.
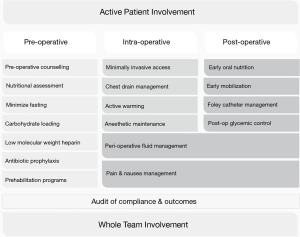
As of October 2018 no official guidelines are in place for the use of the ERAS protocol in esophageal surgery. Over a dozen comparative studies addressed this subject, where the protocol varied in terms of number and types of items used (10). Both the complexity of esophagectomy and the relatively late and limited introduction of minimally invasive approach to this procedure contribute to delayed postoperative recovery. A 2007 meta-analysis revealed significant reductions in non-surgical and pulmonary complications and shorter LOS in ERAS patients (11). However, conclusions from this review were less definitive due to virtually no randomized trials being included. Thus, the benefits of ERAS in esophageal surgery are still debatable (3,4,12,13). Standardization of perioperative enhanced recovery protocols is still under progress, as surgical units modify their components. It is crucial to work out a streamlined, widely applicable ERAS protocol for esophagectomy. The key points in ERAS protocol are presented on Figure 1.
Preoperative period
While some components of ERAS protocol are universal, others require adjustments to the type of the procedure. It is proven that proper nutritional support improves the outcomes of the treatment. Since patients with esophageal cancer are often malnourished, nutritional intervention should be undertaken (14). Available data also shows that frailty rather than age is a major risk factor of perioperative complications (15). This evidence suggests including patient tailored physical activity in the time between diagnosis and the surgical procedure (16-18). Careful communication with the patients is of utter importance. As patients may not be aware of the ERAS protocol components, meticulous explanation is advised (19). Providing information about perioperative treatment also diminishes the patient’s anxiety (20).
One of the pillars of preoperative care is to avoid fasting, which increases insulin-resistance. Insulin-resistance is a major risk factor for infections and cardiovascular complications (21). To minimize the period of fasting, intake of carbohydrate rich fluid 2–3 hours before the procedure is advised. What is important, preoperative fasting delays the time to solid food intake in the postoperative period, thus it is even more important to properly educate the surgical team (5,22).
ERAS protocol don’t advice routine mechanical bowel preparation (MBP). It is said that MBP in fact increases the rate of infections and anastomotic leakage. It may also contribute to Low Anterior Resection syndrome in colorectal surgery (23). However, in some cases exceptions should be made. For example, in very low anterior rectal resections with defunctioning ileostomy to protect the anastomosis. Similar situation can be found regarding esophagectomy with reconstruction of the gastrointestinal tract continuity with transverse colon. In that case MPB is necessary. However, we recommend combining the utilization of MPB with antibiotic administration. Such treatment reduces perioperative complications; however, its quality is low (24,25). It seems that the main cause in morbidity reduction is antibiotic preparation. Although in esophageal surgery with transverse colon reconstruction, MBP could not be avoided (26).
Low Molecular Weight Heparin should be used routinely in all patients. LMWH is as effective as non-fractioned heparin regarding occurrence of deep vein thrombosis (27). Moreover, twice a day administration seems to be even more effective and should be taken into consideration (28).
Operative procedure
Introduction of minimally invasive techniques (MIT) to oesophageal surgery raised the question of whether it should be the method of choice (29-31). Minimally invasive procedures reduce operative trauma, facilitates less postoperative pain and shorter lengths of stay (11,32,33). The biggest available meta-analysis by Yibulayin et al. consisting 57 studies including 15,790 patients proved the superiority of minimally invasive esophagectomy over an open approach in terms of post-operative complications and mortality (34). However, only one of included studies was a randomized control trial (RCT). The data from RCT also support minimally invasive approach. The TIME trial was a RCT involving 5 European hospitals involving 115 patients, investigating cases with resectable, intrathoracic esophageal carcinoma. The study proved, that MIT are superior regarding short-term outcomes with comparable 3-year overall and disease-free survival (35). The MIRO trial included 207 patients from 12 surgical centres. The authors compared open Ivor-Lewis esophagectomy with a hybrid approach in which abdominal step was performed laparoscopically and the thoracic part through thoracotomy. The results preferred hybrid approach in terms of overall postoperative morbidity and especially major pulmonary complications (36). Nevertheless, Rinieri et al. point out that the laparoscopic approach can result in a lower number of harvested lymph nodes, although it had no impact on 5-year overall and disease-free survival (37). Therefore MIT should be an approach of choice for esophageal cancer surgery.
Postoperative care
The ERAS protocol advises the avoidance of routine nasogastric tube insertion, since it decreases patients’ well-being but not perioperative complications (38). A meta-analysis of seven comparative studies by Weijs et al. show no benefit from routine gastric decompression what extends the indications for early removal or full resignation from nasogastric tube in perioperative care (39). According to common belief, in esophageal surgery the tube may be not only inserted to decompress the stomach, but also to protect the anastomosis from excessive stretching. However, there is no evidence to support this theory. On the other hand, nasogastric or nasojejunal tube could be used as a route for early postoperative feeding, which is clearly beneficial (40,41). Moreover, enteral nutrition is better than total parenteral nutrition (40,42). Investigating early oral feeding instead of feeding by tube, Zhang et al. obtained good results with no increased rate of anastomotic leakage (43). Also Sun et al. compared early oral feeding (1 postoperative day, POD) versus late oral feeding (7 POD) and obtained satisfactory results with no increased morbidity but higher quality of life (44).
Postoperative pain impairs multiple components of the ERAS protocol. It delays patient mobilisation, decreases quality of life and increases the rate of pulmonary complications. What should be avoided is opioid-based analgesia, since it depresses the respiratory system and affects negatively on gastrointestinal function recovery (20). Thus, non-steroid anti-inflammatory drugs (NSAID) should be the method of choice. There is still discussion as to whether cyclooxygenase (COX) inhibitors damage gastrointestinal mucosa and due to that increase the anastomotic leakage ratio (45). Meta-analysis indicate that selective inhibitors of COX2 should be used, due to their lower anastomotic leakage rates (45). Alternatives such as local anaesthetic drug injection or epidural analgesia could be considered as an element of multimodal therapy. However, no difference regarding systemic and epidural analgesia was found regarding postoperative pain scores in Visser et al.’s meta-analysis (46).
ERAS advocates the avoidance of the routine use of drainage. Although abdominal drainage could be eliminated, this is not applicable to the thoracic cavity, since pneumothorax requires active removal. Available data suggest that routine abdominal drainage is not necessary and thoracic drains should be removed as fast as it is possible (47). Sato et al. compared early chest drains removal versus late drain removal. The first alternative was found to be safe and not associated with higher rate of pulmonary complications. Moreover patients with late drain removal achieved first mobilisation faster, which is one of the most important components of ERAS protocol (48). Early mobilisation contributes to the decrease of pulmonary complications (49).
There is no need for Foley catheter keeping, as it is the major cause of urinary tract infections (20). Urinary Catheter Removal Guidelines state that the catheter should be removed within 24 hours after the surgery, which decreases not only UTI rate but also the length of stay (50). Some patients may suffer from urine retention after Foley removal. Thus careful diuresis monitoring should be performed to detect the indications for Foley reinsertion.
Rationale for ERAS introduction
Although the evidence for ERAS application in esophageal surgery is limited, ERAS has been proven to be beneficial regarding short and long term outcomes in other disciplines of surgery, confirming its versatility (51,52). ERAS was found to be safe in gastric cancer surgery, improving short term outcomes without increasing morbidity (53,54). The same situation is observed in bariatric surgery, recognized as a high-risk surgery due to multiple co-morbidities and the obesity of the patient (7). More importantly, gastric surgery has the most similarities to esophageal resections, since the anastomosis after resection is within the upper gastrointestinal tract and it should be most vulnerable for introduction of early oral nutrition. Moreover, ERAS decreases the cost of the treatment without comprising the outcomes (55,56). Recent data also show that compliance to ERAS protocol improves survival in patients undergoing colorectal surgery due to cancer (57).
ERAS implementation in esophageal surgery
Until now no clear guidelines existed on which elements of ERAS protocol should be used. Thus the evidence about its influence on postoperative results might be biased. In the Pisarska et al. meta-analysis of 13 trials assessing ERAS in esophageal surgery, the number of items of the protocol varies from 8 to 16 (11). Also, implementation of ERAS resulted only in length of stay and pulmonary specific complication reduction, but not overall morbidity. It improves also the cost effectiveness of the whole treatment (58). There is also substantial lack of randomized control trials. To overcome this limitation, Liu et al. published a study protocol for an up-to date meta-analysis, which should bring new insight into available data. Also, recently ERAS society published recommendations for oesophageal resections (10). This is an opportunity for unification of the protocol, which would make the studies more reliable for comparison and better able from which to draw new conclusions. Nonetheless many items included in the perioperative care are still based on low and moderate level of evidence. Thus the guidelines will require further evaluation and verification.
Conclusions
ERAS significantly improves perioperative outcomes in different branches of surgery. It seems to be beneficial in esophagectomy as well. However, its utilization is still evolving. Surgery specific items require further confirmation, preferably in randomized control trials. Nonetheless, the successful introduction of ERAS into almost every type of surgical procedures (including gastrointestinal surgery, gynecology, urology, orthopaedics and even lung cancer surgery) provides optimism in the further development of this protocol in esophagectomy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg 2002;183:630-41. [Crossref] [PubMed]
- Nygren J, Thacker J, Carli F, et al. Guidelines for perioperative care in elective rectal/pelvic surgery: Enhanced Recovery After Surgery (ERAS((R))) Society recommendations. World J Surg 2013;37:285-305. [Crossref] [PubMed]
- Lassen K, Coolsen MME, Slim K, et al. Guidelines for perioperative care for pancreaticoduodenectomy: Enhanced Recovery After Surgery (ERAS(R)) Society recommendations. World J Surg 2013;37:240-58. [Crossref] [PubMed]
- Gustafsson UO, Scott MJ, Schwenk W, et al. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS(®)) Society recommendations. World J Surg 2013;37:259-84. [Crossref] [PubMed]
- Kisielewski M, Rubinkiewicz M, Pedziwiatr M, et al. Are we ready for the ERAS protocol in colorectal surgery? Wideochir Inne Tech Maloinwazyjne 2017;12:7-12. [Crossref] [PubMed]
- Spanjersberg WR, van Sambeeck JDP, Bremers A, et al. Systematic review and meta-analysis for laparoscopic versus open colon surgery with or without an ERAS programme. Surg Endosc 2015;29:3443-53. [Crossref] [PubMed]
- Małczak P, Pisarska M, Piotr M, et al. Enhanced Recovery after Bariatric Surgery: Systematic Review and Meta-Analysis. Obes Surg 2017;27:226-35. [Crossref] [PubMed]
- Jones EL, Wainwright TW, Foster JD, et al. A systematic review of patient reported outcomes and patient experience in enhanced recovery after orthopaedic surgery. Ann R Coll Surg Engl 2014;96:89-94. [Crossref] [PubMed]
- de Groot JJA, Ament SMC, Maessen JMC, et al. Enhanced recovery pathways in abdominal gynecologic surgery: a systematic review and meta-analysis. Acta Obstet Gynecol Scand 2016;95:382-95. [Crossref] [PubMed]
- Low DE, Allum W, De Manzoni G, et al. Guidelines for Perioperative Care in Esophagectomy: Enhanced Recovery After Surgery (ERAS®) Society Recommendations. World J Surg 2019;43:299-330. [Crossref] [PubMed]
- Pisarska M, Małczak P, Major P, et al. Enhanced recovery after surgery protocol in oesophageal cancer surgery: Systematic review and meta-analysis. PLoS One 2017;12:e0174382. [Crossref] [PubMed]
- Halliday LJ, Markar SR, Doran SLF, et al. Enhanced recovery protocols after oesophagectomy. J Thorac Dis 2017;9:S781-4. [Crossref] [PubMed]
- Li W, Zheng B, Zhang S, et al. Feasibility and outcomes of modified enhanced recovery after surgery for nursing management of aged patients undergoing esophagectomy. J Thorac Dis 2017;9:5212-9. [Crossref] [PubMed]
- Yoshida N, Baba Y, Baba H. Preoperative malnutrition and prognosis after neoadjuvant chemotherapy followed by subsequent esophagectomy. J Thorac Dis 2017;9:3437-9. [Crossref] [PubMed]
- Ørum M, Gregersen M, Jensen K, et al. Frailty status but not age predicts complications in elderly cancer patients: a follow-up study. Acta Oncol 2018;57:1458-66. [Crossref] [PubMed]
- Guinan EM, Dowds J, Donohoe C, et al. The physiotherapist and the esophageal cancer patient: from prehabilitation to rehabilitation. Dis Esophagus 2017;30:1-12. [PubMed]
- Minnella EM, Awasthi R, Loiselle SE, et al. Effect of Exercise and Nutrition Prehabilitation on Functional Capacity in Esophagogastric Cancer Surgery. JAMA Surg 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Sanchez-Lorente D, Navarro-Ripoll R, Guzman R, et al. Prehabilitation in thoracic surgery. J Thorac Dis 2018;10:S2593-600. [Crossref] [PubMed]
- Refai M, Andolfi M, Gentili P, et al. Enhanced recovery after thoracic surgery: patient information and care-plans. J Thorac Dis 2018;10:S512-6. [Crossref] [PubMed]
- Gustafsson UO, Scott MJ, Schwenk W, et al. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Clin Nutr 2012;31:783-800. [Crossref] [PubMed]
- Ljungqvist O., Jonathan E. Rhoads lecture 2011: Insulin resistance and enhanced recovery after surgery. JPEN J Parenter Enteral Nutr 2012;36:389-98. [Crossref] [PubMed]
- Tsang E, Lambert E, Carey S. Fasting leads to fasting: examining the relationships between perioperative fasting times and fasting for symptoms in patients undergoing elective abdominal surgery. Asia Pac J Clin Nutr 2018;27:968-74. [PubMed]
- Nowakowski MM, Rubinkiewicz M, Gajewska N, et al. Defunctioning ileostomy and mechanical bowel preparation may contribute to development of low anterior resection syndrome. Wideochir Inne Tech Maloinwazyjne 2018;13:306-14. [Crossref] [PubMed]
- Moghadamyeghaneh Z, Hanna MH, Carmichael JC, et al. Nationwide Analysis of Outcomes of Bowel Preparation in Colon Surgery. J Am Coll Surg 2015;220:912-20. [Crossref] [PubMed]
- Kiran RP, Murray ACA, Chiuzan C, et al. Combined Preoperative Mechanical Bowel Preparation With Oral Antibiotics Significantly Reduces Surgical Site Infection, Anastomotic Leak, and Ileus After Colorectal Surgery. Ann Surg 2015;262:416-25. [Crossref] [PubMed]
- Klinger AL, Green H, Monlezun DJ, et al. The Role of Bowel Preparation in Colorectal Surgery: Results of the 2012-2015 ACS-NSQIP Data. Ann Surg 2019;269:671-7. [Crossref] [PubMed]
- Akl EA, Kahale LA, Sperati F, et al. Low molecular weight heparin versus unfractionated heparin for perioperative thromboprophylaxis in patients with cancer. Cochrane Database Syst Rev 2014.CD009447. [PubMed]
- Song JQ, Xuan LZ, Wu W, et al. Low molecular weight heparin once versus twice for thromboprophylaxis following esophagectomy: a randomised, double-blind and placebo-controlled trial. J Thorac Dis 2015;7:1158-64. [PubMed]
- van Workum F, Berkelmans GH, Klarenbeek BR, et al. McKeown or Ivor Lewis totally minimally invasive esophagectomy for cancer of the esophagus and gastroesophageal junction: systematic review and meta-analysis. J Thorac Dis 2017;9:S826-33. [Crossref] [PubMed]
- Cuesta MA, Straatman J, van der Peet DL. Comment on: A Propensity Score Matched Analysis of Open Versus Minimally Invasive Transthoracic Esophagectomy in the Netherlands. Ann Surg 2018;268:e74-5. [Crossref] [PubMed]
- Piątkowski J, Jackowski M, Szeliga J. Laparoscopic surgery of esophageal hiatus hernia - single center experience. Wideochirurgia i Inne Tech Maloinwazyjne = Videosurgery Other Miniinvasive Tech 2014;9:13-7.
- Achim F, Constantinoiu S. Recent Advances in Minimally Invasive Esophagectomy. Chirurgia (Bucur) 2018;113:19. [Crossref] [PubMed]
- Zhang X, Yang Y, Ye B, et al. Minimally invasive esophagectomy is a safe surgical treatment for locally advanced pathologic T3 esophageal squamous cell carcinoma. J Thorac Dis 2017;9:2982-91. [Crossref] [PubMed]
- Yibulayin W, Abulizi S, Lv H, et al. Minimally invasive oesophagectomy versus open esophagectomy for resectable esophageal cancer: a meta-analysis. World J Surg Oncol 2016;14:304. [Crossref] [PubMed]
- Straatman J, van der Wielen N, Cuesta MA, et al. Minimally Invasive Versus Open Esophageal Resection. Ann Surg 2017;266:232-6. [Crossref] [PubMed]
- Mariette C, Meunier B, Pezet D, et al. Hybrid minimally invasive versus open oesophagectomy for patients with oesophageal cancer: A multicenter, open-label, randomized phase III controlled trial, the MIRO trial. J Clin Oncol 2015;33:abstr 5.
- Rinieri P, Ouattara M, Brioude G, et al. Long-term outcome of open versus hybrid minimally invasive Ivor Lewis oesophagectomy: a propensity score matched study. Eur J Cardio-Thoracic Surg 2016;51:ezw273.
- Fearon KCH, Ljungqvist O, Von Meyenfeldt M, et al. Enhanced recovery after surgery: a consensus review of clinical care for patients undergoing colonic resection. Clin Nutr 2005;24:466-77. [Crossref] [PubMed]
- Weijs TJ, Kumagai K, Berkelmans GHK, et al. Nasogastric decompression following esophagectomy: a systematic literature review and meta-analysis. Dis Esophagus 2017;30:1-8. [PubMed]
- Berkelmans GH, van Workum F, Weijs TJ, et al. The feeding route after esophagectomy: a review of literature. J Thorac Dis 2017;9:S785-91. [Crossref] [PubMed]
- Cuesta MA. The first randomized controlled trial on early versus late oral feeding after minimally invasive esophagectomy and the ongoing quest for more evidence. J Thorac Dis 2017;9:3635-7. [Crossref] [PubMed]
- Koyanagi K, Tachimori Y. Early oral nutrition plays an active role in enhanced recovery after minimally invasive esophagectomy. J Thorac Dis 2017;9:3598-602. [Crossref] [PubMed]
- Zhang R, Li Y, Liu S, et al. FA01.03: Use of ‘non-tube no fasting’ eras protocol in patients after mie with li’s anastomosis: outcomes in the first 113 patients performed by a surgeon after training course. Dis Esophagus 2018;31:1-2. [Crossref] [PubMed]
- Sun HB, Li Y, Liu XB, et al. Early Oral Feeding Following McKeown Minimally Invasive Esophagectomy. Ann Surg 2018;267:435-42. [Crossref] [PubMed]
- Modasi A, Pace D, Godwin M, et al. NSAID administration post colorectal surgery increases anastomotic leak rate: systematic review/meta-analysis. Surg Endosc 2019;33:879-85. [Crossref] [PubMed]
- Visser E, Marsman M, van Rossum PSN, et al. Postoperative pain management after esophagectomy: a systematic review and meta-analysis. Dis Esophagus 2017;30:1-11. [PubMed]
- Pan H, Hu X, Yu Z, et al. Use of a fast-track surgery protocol on patients undergoing minimally invasive oesophagectomy: preliminary results. Interact Cardiovasc Thorac Surg 2014;19:441-7. [Crossref] [PubMed]
- Sato T, Fujita T, Okada N, et al. Postoperative pulmonary complications and thoracocentesis associated with early versus late chest tube removal after thoracic esophagectomy with three-field dissection: a propensity score matching analysis. Surg Today 2018;48:1020-30. [Crossref] [PubMed]
- Moradian ST, Najafloo M, Mahmoudi H, et al. Early mobilization reduces the atelectasis and pleural effusion in patients undergoing coronary artery bypass graft surgery: A randomized clinical trial. J Vasc Nurs 2017;35:141-5. [Crossref] [PubMed]
- Okrainec A, Aarts MA, Conn LG, et al. Compliance with Urinary Catheter Removal Guidelines Leads to Improved Outcome in Enhanced Recovery After Surgery Patients. J Gastrointest Surg 2017;21:1309-17. [Crossref] [PubMed]
- Offodile AC, Gu C, Boukovalas S, et al. Enhanced recovery after surgery (ERAS) pathways in breast reconstruction: systematic review and meta-analysis of the literature. Breast Cancer Res Treat 2019;173:65-77. [Crossref] [PubMed]
- Miralpeix E, Nick AM, Meyer LA, et al. A call for new standard of care in perioperative gynecologic oncology practice: Impact of enhanced recovery after surgery (ERAS) programs. Gynecol Oncol 2016;141:371-8. [Crossref] [PubMed]
- Li MZ, Wu W, Li L, et al. Is ERAS effective and safe in laparoscopic gastrectomy for gastric carcinoma? A meta-analysis. World J Surg Oncol 2018;16:17. [Crossref] [PubMed]
- Pisarska M, Pędziwiatr M, Major P, et al. Laparoscopic gastrectomy with enhanced recovery after surgery protocol: Single-center experience. Med Sci Monit 2017;23:1421-7. [Crossref] [PubMed]
- Joliat G-R, Labgaa I, Hübner M, et al. Cost-Benefit Analysis of the Implementation of an Enhanced Recovery Program in Liver Surgery. World J Surg 2016;40:2441-50. [Crossref] [PubMed]
- Pędziwiatr M, Wierdak M, Nowakowski M, et al. Cost minimization analysis of laparoscopic surgery for colorectal cancer within the enhanced recovery after surgery (ERAS) protocol: a single-centre, case-matched study. Wideochir Inne Tech Maloinwazyjne 2016;11:14-21. [Crossref] [PubMed]
- Gustafsson UO, Oppelstrup H, Thorell A, et al. Adherence to the ERAS protocol is Associated with 5-Year Survival After Colorectal Cancer Surgery: A Retrospective Cohort Study. World J Surg 2016;40:1741-7. [Crossref] [PubMed]
- Bhandari R, Hao YY. Implementation and Effectiveness of Early Chest Tube Removal during an Enhanced Recovery Programme after Oesophago-gastrectomy. JNMA J Nepal Med Assoc 2015;53:24-7. [Crossref] [PubMed]