
Delayed emptying of the gastric conduit after esophagectomy
Introduction
In 1980 patients with esophageal cancer that underwent curatively intended surgery had an overall 5-year survival below 10% (1). During the following decades, further development of clinical staging, surgery, perioperative care and implementation of neoadjuvant and perioperative oncological treatment modalities led to improved outcomes, with overall 5-year survival being reported in the range of 16–59% (2,3). Prognosis, in terms of survival remains a challenge but the rising number of patients surviving esophagectomy puts other outcome measures in focus as well, such as short and long-term morbidity related to surgery, one of which is the delayed emptying of the gastric conduit after esophagectomy. The reconstruction of the digestive pathway after esophagectomy, by mobilizing a gastric conduit to the thorax and creating an anastomosis to the remaining oral part of the esophagus, is currently the most common procedure (4-6). Delayed emptying of ingested food or fluid to the duodenum has been associated with short term adverse outcomes such as anastomotic leak, pneumonia, longer postoperative ICU and total hospital admission, and in the long term may cause a variety of symptoms, nutritional problems and have a marked effect on quality of life (7-12).
Terms such as gastric outlet obstruction and delayed gastric emptying, have been used in the context of delayed emptying of the gastric conduit. These terms have sometimes been used interchangeably or sometimes using the former in the context of functional tests, and the latter for clinical signs, but these terms are also very well established for conditions unrelated to esophageal surgery (12-15). So, for the purpose of clarity, and to emphasize the unique anatomical and physiological properties of the gastric conduit, we will refer to the condition as delayed gastric conduit emptying (DGCE). The purpose of this article is to provide an up-to-date overview, and to bring to attention some of the knowledge gaps that may be the focus for future research.
Epidemiology of DGCE
Commonly, the incidence of clinically relevant DGCE is considered to be in the range of 10–20% (16-18). Manifestation of symptoms of DGCE has however been reported to occur in over 50% of patients after esophagectomy (9,19-21). Interestingly, in a recent systematic review on the effect of pyloric management after esophageal resection, 25 comparative studies were found that attempted to define postoperative DGCE either based on clinical, radiological or combined clinical and radiological criteria. The overall reported incidence of DGCE was in the range of 2.2–47% (22). In subgroups of the studies, the incidence of delayed emptying was 0–96%. The criteria defining delayed gastric emptying varied, and the study reporting 96% delayed emptying in a subgroup used water soluble contrast swallow at day 4 after surgery with a highly sensitive cut-off limit (23).
In addition to the diversity of diagnostic criteria, the time of diagnosis of DGCE in relation to the time of surgery varies between studies, which also may affect the incidence reported. This has been taken into consideration in some studies focusing on DGCE in the early postoperative period (7,24).
Risk factors of DGCE were studied by Benedix et al. in a retrospective study of 182 patients that underwent esophagectomy. According to the definition of DGCE in the study, 39% developed DGCE. In univariate analysis, pre-existing pulmonary comorbidity was significantly associated with DGCE and postoperative complications of anastomotic leak, and pulmonary complication were also significantly associated with DGCE. The association between anastomotic leak and DGCE has been reported previously (7). In the multivariate analysis female gender was significantly associated with anastomotic leak (10), this finding was however not confirmed in a study by Zhang et al, reporting outcome for 285 consecutive patients with 18.2% overall incidence of DGCE, finding the use of the whole stomach as a conduit the only independent risk factor (25). The effect of different operative strategies on the incidence of DGCE will be further discussed in the treatment section below.
Pathophysiology of DGCE
The native stomach, especially the fundus and proximal corpus, has a role as a reservoir. The native stomach also functions as a pump, with especially the distal corpus, antrum and pylorus grinding and passing forth appropriate amounts of ingested food, reduced to chime and small particles, into the duodenum. Postprandial gastric motility in the proximal, reservoir part, is initially characterized by receptive and adaptive relaxation, mediated by stimulatory vagal input, and intrinsic and vasovagal reflexes to food, stretching the stomach. The gastric pacemaker, situated at the mid-portion of the greater curvature initiates smooth muscle membrane depolarization in waves moving in proximal to distal direction, in a frequency of 3 waves/minute. Myenteric plexus stimuli, triggered by the presence of food, or vagal or humoral stimulation, induce smooth muscle contractions in synchrony with the pacemaker waves. This gives rise to the pumping action of the distal stomach, and the powerful contraction of the distal antrum and pylorus, known as the atrial systole. The pylorus remains mainly contracted during this peristaltic activity, contributing to the grinding effect on food particles, only opening in short periods, allowing chime and small particles to pass to the duodenum. Vagal parasympathetic stimulation is mediated by the excitatory neurotransmitter acetylcholine. Inhibitory sympathetic stimuli are mediated through the lesser and greater splanchnic nerves to the coeliac and mesenteric ganglia and axis along the vessels to the stomach (26-28).
The underlying pathophysiology of DGCE has been studied to some extent, but results have been somewhat contradictory (20). Inherent to the oncological surgical resection is a denervation of the stomach and pylorus, as the vagal nerves are divided, and lymph node dissection disrupts the sympathetic input by the celiac and mesenteric axis to varying degrees. The mobilization of the stomach as an esophageal substitute to the thorax causes a disruption of the native anti-reflux mechanisms of the hiatal structures and angle of His. This renders the patient more susceptible to reflux and regurgitation (20,29). The complex physiology of the motility of the denervated gastric conduit, its recovery in time, and the regulatory functions in play, are not fully understood (30) and in a recent systematic review on the impact of pyloric drainage procedures, Arya et al. emphasized the importance of gaining further knowledge of the anatomy and physiology of the pylorus in the context of DGCE (22). It is likely that the underlying pathophysiological mechanisms behind DGCE may vary to some extent between patients. The predominant cause may be due to a relaxation dysfunction of the pylorus in some patients, dysfunctional peristalsis in some, or a depleted coordination of those functions in others. In addition, ineffective thoraco-abdominal bolus passage may be affected by an unfavorable pressure gradient, or by kinking of the conduit, or, perhaps a combination of those factors altogether. This highly complex combination of potential causal factors behind DGCE might indicate that a more detailed evaluation of the pylorus, gastric conduit peristalsis and pathway on a case by case basis is necessary to identify the correct treatment for the individual patient with DGCE (22). However, the common denominator is the symptomatic delayed passage of gastric content.
Other factors that may affect the optimal passing time of food, are the level of anastomosis, anastomotic technique, size of the gastric conduit (31), and the topographic pathway of the conduit (32). Peristaltic movement of the gastric conduit has been shown, in general, to improve with time after surgery (30,33-35). Other factors related to the postoperative phase, such as post-surgical bowel paralysis, patient mobilization after surgery and onset of per oral diet intake, may also affect early postoperative gastric conduit emptying. It is rational to believe that DGCE in the immediate post-surgical weeks will have a different symptom panorama, and that factors such as postoperative bowel paralysis will affect the physiology and function of the gastric conduit. It may also be rational to divide DGCE into two temporal entities, considering early and late DGCE as separate conditions, with different underlying pathophysiology, different symptoms and requiring different diagnostic modalities and perhaps also treatment. Some authors have acknowledged such a distinction, but no consensus has yet been made public (24,36).
Symptoms of DGCE
Several studies exist on different aspects of DGCE, mainly focusing on prevention or treatment options, and even though some have described diagnostic criteria for the purpose of the study, symptoms attributed to DGCE seem to be derived mainly from clinical experience, as no study has been published with the aim to systematically describe the symptoms of DGCE. The reported symptoms of DGCE differ between studies, but symptoms commonly reported are listed in Table 1 (8,11,15,21,25,32,37-39). Those symptoms may overlap to a variable extent, and some of the symptoms may not be particularly specific to DGCE, but instead reflect symptoms normally occurring after esophagectomy. An obvious difficulty in this endeavor is the lack of a uniform definition and diagnostic criteria for DGCE. The symptoms of DGCE need to be studied in well-defined patient populations and thereafter validated in separate cohorts. In addition, useful symptom grading tools need to be developed for severity grading of DGCE (10,20,22). As an example, in a study on early DGCE comparing per-operative digital fracture dilatation of the pylorus to no pyloric intervention, Deng et al. used a questionnaire with 6 gastric, and 3 esophageal symptoms to indicate symptom severity for early DGCE. On day 10 after surgery, the patients were asked to report the presence of each symptom resulting in a numeric value between 0 and 3. There was a significant difference in total score of gastric symptoms in favor of the digital fracture group (24).

Full table
Diagnostic modalities in DGCE, the role of radiology, functional studies and endoscopy
Several diagnostic modalities have been used in the diagnosis and treatment response evaluation of DGCE. These include upper GI endoscopy, chest X-ray, gastric scintigraphy, water-soluble and barium contrast studies and paracetamol absorption test. As with symptoms of DGCE, evaluation of which diagnostic modalities are best suited for the diagnosis of DGCE has been given little attention. AND there is to date no gold standard functional radiological method for DGCE diagnosis and severity grading. In fact, not even the normal post-esophagectomy gastric conduit emptying pattern, using the most common functional radiological methods, are well described.
Endoscopy is a valuable tool in excluding differential diagnoses such as anastomotic stricture or other causes of upper GI obstruction. Some authors consider that endoscopic signs, such as the presence of residual food in the stomach despite adequate fasting, or a narrow pyloric orifice, give support to the diagnosis (7,15). However, generally accepted cut-off values regarding ingested and residual food amounts and time since last meal, in order to quantify endoscopically detected food retention, is not available.
Chest X-ray is commonly performed in the postoperative period after esophagectomy, and the presence of air-fluid levels or dilatation of the gastric conduit has been considered to support the diagnosis of DGCE (7,16).
Gastric scintigraphy has been the investigation of choice in most of the comparative studies investigating the effect of pyloric drainage on DGCE (22). In a paper published by Kim et al. in 2007, on the effects of pyloric balloon dilatation for DGCE, a gastric scintigraphy was routinely performed after surgery. A technetium labeled meal was given, and DGCE was considered diagnosed if 50% or more of the peak activity was present 180 minutes after ingestion. Nineteen patients had a follow up scintigraphy after dilatation and 68% improved their gastric emptying (40). In some contrast, Deng et al. defined DGCE to be present if 10% of a radioactive meal was present 4 hours after ingestion (24). Few studies attempt such a formal definition of cut-off values for the diagnosis of DGCE on gastric scintigraphy, and most define a normal rate of emptying as the rate observed in the patient group not requiring an intervention for DGCE. Gastric scintigraphy is not widely available as a standard clinical examination, and is comparatively time consuming, which may prevent this modality from becoming a standard tool for widespread clinical use.
Other studies have used upper GI water soluble contrast swallow to evaluate the occurrence of DGCE (19,41). An advantage of this method is its widespread use in clinical practice and general availability. A weakness may be that this method rather measures the emptying ability for fluid and may give less information on emptying ability for solid food. A more viscous contrast medium commonly available is Barium contrast. There is however evidence that aspiration of Barium contrast may cause more severe lung injury, compared to water soluble contrast media such as diatrizoic acid (Gastrografin) (42).
The paracetamol absorption test has been validated as a method to measure gastric emptying rate (43). In a study on patients after gastric conduit reconstruction the paracetamol absorption test was compared to gastric scintigraphy, as a gold standard. Patients received a semisolid meal labeled with 40 MBq of 99mTc-Nanocoll that even contained paracetamol 20 mg/kg patient weight. Paracetamol emptying test had a good concordance to the 25% and 50% gastric conduit emptying rate of the labeled meal according to scintigraphy (35).
In the scarce available data there is a lack of correlation between symptom severity of DGCE and functional radiology (40). This may be attributed to the fact that no consensus has been reached regarding diagnostic criteria and cardinal symptoms of DGCE. Further studies are required correlating symptom severity measures to functional radiology study results.
Diagnosis of DGCE
Currently, the diagnostic criteria for DGCE vary between centers (22). The results of different observational studies and clinical trials are therefore difficult to compare (20). The need for a generally accepted definition of DGCE and a consensus regarding diagnostic criteria has been identified by many researchers in the field (10,20-22,32,44-46).
In addition to the disparity of definition and diagnostic criteria of DGCE, few studies attempt to classify DGCE in relation to how long time has passed since esophageal surgery. The clinical settings and the condition of patients will differ in the early and late phase. This will affect both the symptom panorama, as well as which investigative and treatment modalities will be appropriate for early vs. late onset DGCE. For these reasons, it may be reasonable to address early and late DGCE separately, with different diagnostic criteria and different treatment options.
For DGCE after the immediate post-surgical period, the cardinal symptoms need to be established, and a symptom grading instrument and a functional radiology method, for DGCE diagnosis and severity assessment, need to be established and subsequently validated.
Treatment strategies of DGCE
Regarding prevention of DGCE after esophagectomy, many surgeons have performed pyloric drainage procedures, initially based on experience from pyloric dysfunction after elective vagotomy in the treatment of ulcer disease (47). The benefit of perioperative pyloromyotomy or pyloroplasty, in the context of esophagectomy, continues to be a matter of debate. Urschel et al. performed a meta-analysis of trials comparing perioperative surgical drainage to no drainage and found that drainage procedures resulted in significantly lower relative risk of DGCE as defined by the original articles or by impairment of gastric emptying on the 1-week postoperative contrast study, while no effect was found on outcome measures such as anastomotic leak or pulmonary complications (21). In a large retrospective analysis, Palmes et al. found no significant effect of drainage procedures on early postoperative outcome, including DGCE according to 4th postoperative day gastrographin swallow while on follow-up endoscopy at 12 months, presence of esophagitis and bile reflux was significantly increased in the drainage group (19). In a more recent systematic review Arya et al., analyzing 6 studies comparing various pyloric drainage procedures, including Botulinum toxin injections and digital pyloric fracture as well, found no significant differences in gastric emptying on functional studies, while the main conclusion was that due to the imprecise and heterogeneous definitions of DGCE, an intra-study comparison was not feasible (22).
Intra-pyloric injection of botulinum toxin during esophageal surgery has been proposed as a promising alternative to mechanical disruption of the pylorus, as a gastric drainage procedure. In theory, botulinum toxin injection could relax the pylorus during the early postoperative period, reducing the rate of early DGCE, without the permanent effects of surgical drainage procedures, with potentially increased bile reflux and dumping symptoms (23,32,48). Controlled studies have indeed shown promising results, with incidence of early DGCE lower than those where no drainage procedures have been performed and comparable to those where surgical drainage has been performed (23,38). However, in long-term results reported by Eldaif et al., patients who received intra-pyloric botulinum toxin injection during surgery had significantly more reflux symptoms and required more frequent post-operative endoscopic pylorus dilatations and more use of promotility agents, compared to controls that received other pyloric drainage procedures during esophagectomy (49).
The effects of other variations in surgical technique during esophagectomy were analyzed in a recent systematic review by Akkerman et al. The study found that gastric tube reconstruction was associated with a significantly reduced risk of DGCE compared to the use of whole stomach. In terms of anastomotic site, or reconstructive route, the authors concluded that available evidence is not conclusive regarding the effect on DGCE (32).
In the past decades, minimally invasive procedures have become increasingly common, and pyloric drainage procedures have commonly been omitted from the standard operative protocol. This has to some extent moved focus from preventive interventions to post-operative DGCE treatment strategies. Several studies have been published indicating the effect of balloon dilatation of the pylorus, with balloon diameters often ranging from 12–22 mm (15,40). In a pilot study, Ericsson et al. reported on the safety and feasibility of a fluoroscopy guided large diameter balloon dilatation of the pylorus, using either a 30 or 35 mm pneumatic dilatation balloon, conventionally used to treat achalasia (46). Maus et al. reported the effect of either a through the scope dilatation balloon, or a 30 or 35 mm achalasia balloon, with a 2.5-fold lower risk for re-dilatation with the use of the large diameter balloon (17). Endoscopic balloon dilatation has also been studied as a preventive measure of DGCE prior to surgery (50). Successful cases of gastric per-oral endoscopic myotomy (G-POEM) of the pylorus have been reported, however larger studies are needed to determine its clinical applicability (51,52). Another recently emerged alternative for treatment of intractable DGCE is the implantation of a neurostimulator in the gastric conduit, however, at present only a few case reports are available (53,54).
Less invasive treatment strategies should be considered prior or parallel to interventions. As in general for patients after esophagectomy, the daily need of calories and nutrition must be provided for, and it is mandatory that the patients get meticulous counseling, using a team approach, including surgeons, dieticians and contact nurses. It is furthermore very important that supplemental nutrition is provided as needed until the problem of DGCE is resolved. Pharmacological strategies include prokinetic agents, and other pharmacological symptom amelioration, such as adjusting the dose of proton pump inhibitors, or adding antacids, to reduce the symptoms of reflux.
There is evidence that the macrolide antibiotic erythromycin acts as an agonist to motilin receptors, found in the smooth muscle of the antrum and pylorus (55,56). In a small prospective non-randomized trial, Hill et al. found that oral erythromycin 250 mg thrice daily for three days improved gastric emptying significantly on scintigraphy 3 months postoperatively compared to pre-treatment, and additionally the remaining isotope activity at 4 hours was similar to that in normal controls (34). In a randomized trial, Burt et al. showed that intravenous erythromycin significantly improved gastric emptying on scintigraphy 11 days after esophageal surgery with pyloric drainage (55). The early postsurgical effect of erythromycin was further supported by data published by Collard et al. (57). Nakabayashi et al investigated the effect of erythromycin on the fasting motor activity of gastric tubes of patients 3, 12 and 24 months after esophagectomy, and found that erythromycin was able to induce periods of strong antro-pyloric peristaltic contractions (phase III of the migrating motor complex) in 44% of the patients 12 months or longer after surgery, but no effect was observed in the group of patients investigated 3 months after surgery. This study indicates that the conduit may need more time to recover before the effect of erythromycin can be detected (30). There are indications that the effect of erythromycin is clearer for patients with a whole stomach reconstruction (33). To date, all studies studying erythromycin and gastric conduit emptying have included small patient samples. Larger clinical trials with well-defined diagnostic criteria for DGCE, assessing the effect of erythromycin on DGCE symptoms and functional conduit emptying are needed to clarify the role of erythromycin in the treatment of DGCE after esophagectomy. Importantly, potential serious side effects of erythromycin, such as cardiac arrhythmias, should be taken into consideration, as well as the potential effects on gastrointestinal microbiota of long-term antibiotic treatment (20). An overview of selected articles addressing treatment strategies of DGCE is presented in Table 2.
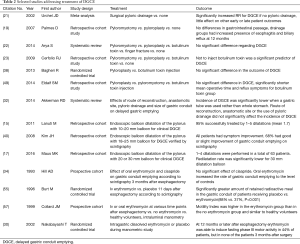
Full table
Conclusions
In the context of the lower postoperative mortality and improved long-term survival recently observed after curatively intended surgery for esophageal cancer, further attention is drawn to the functional outcome of the surgically altered anatomy after esophagectomy. The occurrence of DGCE puts the patient at risk for malnutrition and impaired health-related quality of life. The underlying pathophysiology is not fully understood and needs to be further clarified in order to provide a basis for optimized treatment strategies.
In order to facilitate DGCE research and implementation of research results, in clinical practice, with the objective to reduce morbidity and improve quality of life for patients after esophagectomy, we propose the following actions as the highest priorities:
- To define widely accepted diagnostic criteria for DGCE in an expert consensus process.
- To establish a symptom severity tool, in order to be able to evaluate and compare DGCE symptoms in future research, in an expert consensus process.
- To define and subsequently validate the functional radiological gastric emptying patterns associated with DGCE after esophagectomy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Earlam R, Cunha-Melo JR. Oesophageal squamous cell carcinoma: I. A critical review of surgery. Br J Surg 1980;67:381-90. [Crossref] [PubMed]
- Courrech Staal EF, Aleman BM, Boot H, et al. Systematic review of the benefits and risks of neoadjuvant chemoradiation for oesophageal cancer. Br J Surg 2010;97:1482-96. [Crossref] [PubMed]
- Mariette C, Taillier G, Van Seuningen I, et al. Factors affecting postoperative course and survival after en bloc resection for esophageal carcinoma. Ann Thorac Surg 2004;78:1177-83. [Crossref] [PubMed]
- Davis PA, Law S, Wong J. Colonic interposition after esophagectomy for cancer. Arch Surg 2003;138:303-8. [Crossref] [PubMed]
- Skinner DB. Esophageal reconstruction. Am J Surg 1980;139:810-4. [Crossref] [PubMed]
- Goldstraw P, Bach P. Gastric emptying after oesophagectomy as assessed by plasma paracetamol concentrations. Thorax 1981;36:493-6. [Crossref] [PubMed]
- Sutcliffe RP, Forshaw MJ, Tandon R, et al. Anastomotic strictures and delayed gastric emptying after esophagectomy: incidence, risk factors and management. Dis Esophagus 2008;21:712-7. [Crossref] [PubMed]
- Gockel I, Gonner U, Domeyer M, et al. Long-term survivors of esophageal cancer: disease-specific quality of life, general health and complications. J Surg Oncol 2010;102:516-22. [Crossref] [PubMed]
- McLarty AJ, Deschamps C, Trastek VF, et al. Esophageal resection for cancer of the esophagus: long-term function and quality of life. Ann Thorac Surg 1997;63:1568-72. [Crossref] [PubMed]
- Benedix F, Willems T, Kropf S, et al. Risk factors for delayed gastric emptying after esophagectomy. Langenbecks Arch Surg 2017;402:547-54. [Crossref] [PubMed]
- Anandavadivelan P, Wikman A, Johar A, et al. Impact of weight loss and eating difficulties on health-related quality of life up to 10 years after oesophagectomy for cancer. Br J Surg 2018;105:410-8. [Crossref] [PubMed]
- Deldycke A, Van Daele E, Ceelen W, et al. Functional outcome after Ivor Lewis esophagectomy for cancer. J Surg Oncol 2016;113:24-8. [Crossref] [PubMed]
- Coleski R, Baker JR, Hasler WL. Endoscopic Gastric Food Retention in Relation to Scintigraphic Gastric Emptying Delays and Clinical Factors. Dig Dis Sci 2016;61:2593-601. [Crossref] [PubMed]
- Malleo G, Crippa S, Butturini G, et al. Delayed gastric emptying after pylorus-preserving pancreaticoduodenectomy: validation of International Study Group of Pancreatic Surgery classification and analysis of risk factors. HPB (Oxford) 2010;12:610-8. [Crossref] [PubMed]
- Lanuti M, DeDelva P, Morse CR, et al. Management of delayed gastric emptying after esophagectomy with endoscopic balloon dilatation of the pylorus. Ann Thorac Surg 2011;91:1019-24. [Crossref] [PubMed]
- Lanuti M, de Delva PE, Wright CD, et al. Post-esophagectomy gastric outlet obstruction: role of pyloromyotomy and management with endoscopic pyloric dilatation. Eur J Cardiothorac Surg 2007;31:149-53. [Crossref] [PubMed]
- Maus MK, Leers J, Herbold T, et al. Gastric Outlet Obstruction After Esophagectomy: Retrospective Analysis of the Effectiveness and Safety of Postoperative Endoscopic Pyloric Dilatation. World J Surg 2016;40:2405-11. [Crossref] [PubMed]
- Fritz S, Feilhauer K, Schaudt A, et al. Pylorus drainage procedures in thoracoabdominal esophagectomy - a single-center experience and review of the literature. BMC Surg 2018;18:13. [Crossref] [PubMed]
- Palmes D, Weilinghoff M, Colombo-Benkmann M, et al. Effect of pyloric drainage procedures on gastric passage and bile reflux after esophagectomy with gastric conduit reconstruction. Langenbecks Arch Surg 2007;392:135-41. [Crossref] [PubMed]
- Poghosyan T, Gaujoux S, Chirica M, et al. Functional disorders and quality of life after esophagectomy and gastric tube reconstruction for cancer. J Visc Surg 2011;148:e327-35. [Crossref] [PubMed]
- Urschel JD, Blewett CJ, Young JE, et al. Pyloric drainage (pyloroplasty) or no drainage in gastric reconstruction after esophagectomy: a meta-analysis of randomized controlled trials. Dig Surg 2002;19:160-4. [Crossref] [PubMed]
- Arya S, Markar SR, Karthikesalingam A, et al. The impact of pyloric drainage on clinical outcome following esophagectomy: a systematic review. Dis Esophagus 2015;28:326-35. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Canon CL, et al. Is botulinum toxin injection of the pylorus during Ivor Lewis esophagogastrectomy the optimal drainage strategy? J Thorac Cardiovasc Surg 2009;137:565-72. [Crossref] [PubMed]
- Deng B, Tan QY, Jiang YG, et al. Prevention of early delayed gastric emptying after high-level esophagogastrostomy by "pyloric digital fracture". World J Surg 2010;34:2837-43. [Crossref] [PubMed]
- Zhang L, Hou SC, Miao JB, et al. Risk factors for delayed gastric emptying in patients undergoing esophagectomy without pyloric drainage. J Surg Res 2017;213:46-50. [Crossref] [PubMed]
- O'Connor A, O'Morain C. Digestive function of the stomach. Dig Dis 2014;32:186-91. [Crossref] [PubMed]
- Ramkumar D, Schulze KS. The pylorus. Neurogastroenterol Motil 2005;17 Suppl 1:22-30. [Crossref] [PubMed]
- Rostas JW 3rd, Mai TT, Richards WO. Gastric motility physiology and surgical intervention. Surg Clin North Am 2011;91:983-99. [Crossref] [PubMed]
- Donington JS. Functional conduit disorders after esophagectomy. Thorac Surg Clin 2006;16:53-62. [Crossref] [PubMed]
- Nakabayashi T, Mochiki E, Garcia M, et al. Gastropyloric motor activity and the effects of erythromycin given orally after esophagectomy. Am J Surg 2002;183:317-23. [Crossref] [PubMed]
- Bemelman WA, Taat CW, Slors JF, et al. Delayed postoperative emptying after esophageal resection is dependent on the size of the gastric substitute. J Am Coll Surg 1995;180:461-4. [PubMed]
- Akkerman RD, Haverkamp L, van Hillegersberg R, et al. Surgical techniques to prevent delayed gastric emptying after esophagectomy with gastric interposition: a systematic review. Ann Thorac Surg 2014;98:1512-9. [Crossref] [PubMed]
- Collard JM, Romagnoli R, Otte JB, et al. The denervated stomach as an esophageal substitute is a contractile organ. Ann Surg 1998;227:33-9. [Crossref] [PubMed]
- Hill AD, Walsh TN, Hamilton D, et al. Erythromycin improves emptying of the denervated stomach after oesophagectomy. Br J Surg 1993;80:879-81. [Crossref] [PubMed]
- Djerf P, Brundin M, Bajk M, et al. Validation of the paracetamol absorption test for measuring gastric tube emptying in esophagectomized patients versus gold standard scintigraphy. Scand J Gastroenterol 2015;50:1339-47. [Crossref] [PubMed]
- Martin JT, Federico JA, McKelvey AA, et al. Prevention of delayed gastric emptying after esophagectomy: a single center's experience with botulinum toxin. Ann Thorac Surg 2009;87:1708-13; discussion 1713-4.
- Antonoff MB, Puri V, Meyers BF, et al. Comparison of pyloric intervention strategies at the time of esophagectomy: is more better? Ann Thorac Surg 2014;97:1950-7; discussion 1657-8.
- Bagheri R, Fattahi SH, Haghi SZ, et al. Botulinum toxin for prevention of delayed gastric emptying after esophagectomy. Asian Cardiovasc Thorac Ann 2013;21:689-92. [Crossref] [PubMed]
- Burrows WM. Gastrointestinal function and related problems following esophagectomy. Semin Thorac Cardiovasc Surg 2004;16:142-51. [Crossref] [PubMed]
- Kim JH, Lee HS, Kim MS, et al. Balloon dilatation of the pylorus for delayed gastric emptying after esophagectomy. Eur J Cardiothorac Surg 2008;33:1105-11. [Crossref] [PubMed]
- Fok M, Cheng SW, Wong J. Pyloroplasty versus no drainage in gastric replacement of the esophagus. Am J Surg 1991;162:447-52. [Crossref] [PubMed]
- Siddiqui MT, Litts JK, Cheney DM, et al. The effect of aspirated barium sulfate, iodixanol, and diatrizoic acid on survival and lung injury in a lagomorph model. Laryngoscope 2017;127:E148-52. [Crossref] [PubMed]
- Medhus AW, Lofthus CM, Bredesen J, et al. Gastric emptying: the validity of the paracetamol absorption test adjusted for individual pharmacokinetics. Neurogastroenterol Motil 2001;13:179-85. [Crossref] [PubMed]
- Gaur P, Swanson SJ. Should we continue to drain the pylorus in patients undergoing an esophagectomy? Dis Esophagus 2014;27:568-73. [Crossref] [PubMed]
- Boshier PR, Adam ME, Doran S, et al. Effects of intraoperative pyloric stretch procedure on outcomes after esophagectomy. Dis Esophagus 2018;31. [Crossref] [PubMed]
- Ericson J, Sunde B, Lindblad M, et al. Large-diameter (30-35 mm) pneumatic balloon dilatation of the pylorus in patients with gastric outlet obstruction symptoms after esophagectomy. Scand J Surg 2013;102:83-6. [Crossref] [PubMed]
- Dragstedt LR, Camp EH. Follow-up of gastric vagotomy alone in the treatment of peptic ulcer. Gastroenterology 1948;11:460-5. [PubMed]
- Kent MS, Pennathur A, Fabian T, et al. A pilot study of botulinum toxin injection for the treatment of delayed gastric emptying following esophagectomy. Surg Endosc 2007;21:754-7. [Crossref] [PubMed]
- Eldaif SM, Lee R, Adams KN, et al. Intrapyloric botulinum injection increases postoperative esophagectomy complications. Ann Thorac Surg 2014;97:1959-64; discussion 1964-5.
- Swanson EW, Swanson SJ, Swanson RS. Endoscopic pyloric balloon dilatation obviates the need for pyloroplasty at esophagectomy. Surg Endosc 2012;26:2023-8. [Crossref] [PubMed]
- Malik Z, Kataria R, Modayil R, et al. Gastric Per Oral Endoscopic Myotomy (G-POEM) for the Treatment of Refractory Gastroparesis: Early Experience. Dig Dis Sci 2018;63:2405-12. [Crossref] [PubMed]
- Chung H, Dallemagne B, Perretta S, et al. Endoscopic pyloromyotomy for postesophagectomy gastric outlet obstruction. Endoscopy 2014;46 Suppl 1 UCTN:E345-6.
- Asti E, Lovece A, Bonavina L. Thoracoscopic Implant of Neurostimulator for Delayed Gastric Conduit Emptying After Esophagectomy. J Laparoendosc Adv Surg Tech A 2016;26:299-301. [Crossref] [PubMed]
- Salameh JR, Aru GM, Bolton W, et al. Electrostimulation for intractable delayed emptying of intrathoracic stomach after esophagectomy. Ann Thorac Surg 2008;85:1417-9. [Crossref] [PubMed]
- Burt M, Scott A, Williard WC, et al. Erythromycin stimulates gastric emptying after esophagectomy with gastric replacement: a randomized clinical trial. J Thorac Cardiovasc Surg 1996;111:649-54. [Crossref] [PubMed]
- Peeters T, Matthijs G, Depoortere I, et al. Erythromycin is a motilin receptor agonist. Am J Physiol 1989;257:G470-4. [PubMed]
- Collard JM, Romagnoli R, Otte JB, et al. Erythromycin enhances early postoperative contractility of the denervated whole stomach as an esophageal substitute. Ann Surg 1999;229:337-43. [Crossref] [PubMed]


