
KEYNOTE-024: goodbye to chemotherapy?
In the last 2 years the therapeutic landscape of non-small cell lung cancer (NSCLC) has seen a rapid shift with the introduction of immunotherapy into treatment strategy. Studies have demonstrated superior overall survival (OS) for monoclonal antibody therapy, directed against either the PD-1 receptor [nivolumab (1,2) and pembrolizumab (3)] or its ligand [PD-L1, atezolizumab (4,5)], when compared with docetaxel chemotherapy.
Keynote-024 trial
In the first-line setting, the open-label, randomized, phase III KEYNOTE-024 trial, showed that pembrolizumab was more effective if compared with platinum-based chemotherapy in NSCLC patients with high levels of PD-L1 expression [tumor proportion score (TPS) of 50% or higher] (6).
Five hundred (30.2%) out of tumor samples from 1,653 patients were strongly positive for PD-L1 expression (TPS ≥50%). Three-hundreds and five patients out of 500 were randomized to receive pembrolizumab (n=154) or platinum-based chemotherapy (n=151). At second interim analysis, pembrolizumab showed a significant superior progression-free survival (PFS) and OS if compared with standard chemotherapy, meeting the primary objective of the trial. Therefore, the trial was prematurely stopped in order to allow patients receiving chemotherapy to cross over to the pembrolizumab arm. In fact, the difference of PFS and OS in favor of pembrolizumab was statistically and clinically significant. Despite 44% of patients made a crossover from chemotherapy to immunotherapy, pembrolizumab showed a significant improvement in OS [hazard ratio (HR) =0.60, P=0.005]. Furthermore, pembrolizumab showed higher response rates (44.8% vs. 27.8%, respectively) and a longer duration of response with also a better toxicity profile than chemotherapy.
Brahmer et al. also reported the prespecified analysis on patient-reported outcomes (PROs) (7). In that study, pembrolizumab yields better health-related quality of life than platinum-based chemotherapy. Study results showed that at week 15, patients in the pembrolizumab group had experienced an increase in QLQ-C30 mean score of 6.9 points, whereas those in the chemotherapy group had experienced a decrease in QLQ-C30 mean score of 0.9 points (P=0.002).
Furthermore, patients enrolled in the pembrolizumab group had a significant longer time to deterioration as revealed by the QLQ-LC13 with regard to cough, chest pain and dyspnea, which was defined as a reduction in the score for at least 1 of these symptoms by at least 10 points and confirmed by a second reduction of that magnitude (HR =0.66; P=0.029).
Patients receiving pembrolizumab also had more favorable changes in scores on individual QLQ-C30 scales for functioning and symptoms and in QLQ-LC13 scores for specific symptoms.
Based on these data, pembrolizumab has been proposed as a new standard first-line treatment for advanced NSCLC with a PD-L1 expression TPS of greater than or equal to 50%.
It is likely that patients with metastatic NSCLC overexpressing PD-L1 will represent a new subgroup, candidate to immunotherapy. However, different results were obtained with nivolumab in first-line treatment. The CheckMate-026 trial analyzed the efficacy of nivolumab in the first-line setting vs. with platinum-based doublet chemotherapy in patients with advanced NSCLC with positive PD-L1. (8)
The primary endpoint of the trial was PFS in patients with PD-L1 ≥5%. Five hundred and forty-one patients were randomized to receive either nivolumab 3 mg/kg intravenously every 2 weeks or the investigator’s choice of chemotherapy, according to cancer histotype. Nevertheless, nivolumab did not reach its primary endpoint since no benefit in term of PFS was shown over standard chemotherapy.
So why has nivolumab failed to be superior to chemotherapy in first-line NSCLC?
The results of KEYNOTE-024 and CheckMate-026 trials were discordant and this may reflect the differences in study design and in particular the eligibility criteria. In fact, a more stringent PD-L1 expression cut-off was included in the pembrolizumab trial (50%) if compared to the nivolumab study (5%). Furthermore, the two trials showed differences in the PD-L1 analyses.
Overall, data from these two trials seem to confirm a correlation between the intensity of PD-L1 expression and the clinical benefit obtained.
PD-L1 testing
Therefore, it seems now crucial to investigate also within the clinical practice, newly diagnosed advanced NSCLC patients not only for driver mutations such as EGFR, ALK and ROS-1 but also for PD-L1 expression. Nevertheless, some outstanding questions that need to be further addressed still remain.
The first concern regards PD-L1 testing. It is known that there are many variables in the immunohistochemistry (IHC) assays. Among the others:
- The time intervening between sample collection and treatment with checkpoint inhibitor;
- Different PD-L1 antibodies have been used till now in the various trials and currently no validated antibody for IHC is present;
- PD-L1 expression is heterogeneous;
- PD-L1 expression can change over time and it can be also induced by IFN-γ during disease progression and treatment.
Furthermore, in many patients, cancer diagnosis is performed on cytological samples and no histological samples are available. It is clear that these issues may have an impact on the possibility of achieving a detailed molecular characterization with an impact on selecting the most appropriate therapeutic strategy and on outcome.
Immunotherapy plus chemotherapy
The treatment scenario may also change in the near future, since further studies are combining immunotherapy to chemotherapy or different immunotherapy drugs. Table 1 summarizes trials of immunotherapy in NSCLC.
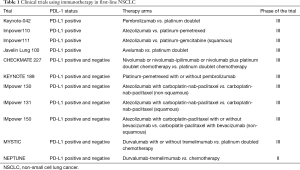
Full table
The KEYNOTE-189 is a phase III trial enrolling 616 patients with advanced, PD-L1-unselected, non-squamous NSCLC, who were randomized in a 2:1 ratio to chemotherapy (platinum including cisplatin or carboplatin with pemetrexed) with pembrolizumab or placebo (9). Results showed that 12-month OS, which was one of the primary endpoints, was 69% in patients receiving pembrolizumab and chemotherapy, vs. 49% for those receiving chemotherapy and placebo (HR =0.49, 95% CI: 0.38–0.64). The other primary endpoint was median PFS that was also significantly improved with the addition of pembrolizumab (8.8 vs. 4.9 months for those receiving chemotherapy and placebo; HR =0.52, 95% CI: 0.43–0.64). OS at 12-month, with and without pembrolizumab, remained in favor of patients receiving pembrolizumab also in subgroup of patients according to PD-L1 expression: it was 73% vs. 48% in patients with ≥50% PD-L1 expression; 72% vs. 51% when PD-L1 expression was between 1% and 50%, and 62% vs. 52% for those with PD-L1 expression <1%, respectively. Toxicity was not significantly increased in the pembrolizumab-combination group.
The KEYNOTE-407 is another phase III trial enrolling 559 patients with PD-L1-unselected, treatment-naïve, advanced squamous NSCLC, who were randomized to receive chemotherapy (carboplatin with either paclitaxel or nab-paclitaxel) with either pembrolizumab or placebo in a 1:1 ratio (10). Median OS was 15.9 months among patients receiving chemotherapy plus pembrolizumab, vs.11.3 months for those undergoing chemotherapy and placebo (HR =0.64, 95% CI: 0.49–0.85). The other primary endpoint was median PFS, which was also improved in the pembrolizumab arm: 6.4 vs. 4.8 months (HR =0.56, 95% CI: 0.45–0.70).
A trend of improvement in median OS and PFS was also shown according to tumor PD-L1 expression. Again, no significant increase of toxicity was observed adding pembrolizumab.
Another trial, the IMpower 150, randomly assigned 1202 patients with PD-L1-unselected, advanced, non-squamous NSCLC to first-line chemotherapy (carboplatin and paclitaxel) combined with either atezolizumab (ACP), atezolizumab plus bevacizumab (ABCP), or bevacizumab (BCP) (11). Coprimary endpoints of PFS and OS for ABCP vs. BCP were met, favoring the addition of atezolizumab to bevacizumab and chemotherapy.
The IMpower 131 trial randomized 683 patients with PD-L1-unselected, advanced, squamous NSCLC to frontline chemotherapy (carboplatin and nab-paclitaxel) alone or combined with atezolizumab (12). In preliminary results, at a median follow-up of 17 months, those assigned to atezolizumab and chemotherapy experienced an improved PFS (6.3 vs. 5.6 months; HR =0.7, 95% CI: 0.60–0.85) relative to those receiving chemotherapy alone. Improvements were seen in all PD-L1-positive subgroups, but not in the PD-L1-negative subgroup.
In the overall population, interim OS results were not significantly different between those receiving atezolizumab and chemotherapy vs. chemotherapy alone (14.0 vs. 13.9 months). While an OS benefit was seen among those with PD-L1-high tumors (23.6 vs. 14.1 months; HR =0.56, 95% CI: 0.32–0.99), there was a trend toward worsened survival with the addition of atezolizumab in the PD-L1-low group for unknown reasons. Among those with PD-L1-negative tumors, OS was 13.8 vs. 12.5 months, with and without the addition of atezolizumab, respectively (HR =0.86, 95% CI: 0.65–1.15).
Immunotherapy combinations
Another field of research, is to combine different immunotherapeutic drugs. The CheckMate 227 is a multi-part trial, randomizing patients with advanced, untreated NSCLC to histology-based, platinum-doublet chemotherapy; nivolumab plus ipilimumab; or either nivolumab monotherapy (for PD-L1 ≥1%) or nivolumab plus chemotherapy (for PD-L1 <1%) (13). Results from part 1 of this study comparing nivolumab plus ipilimumab to chemotherapy in patients with known tumor mutational burden (TMB) have been reported. Of 679 evaluable patients, 299 (44%) had tumors with high TMB, defined as >10 mutations per megabase. PFS in patients with high TMB was longer with nivolumab plus ipilimumab than chemotherapy, irrespective of PD-L1 expression level, with median PFS of 7.2 months and 1-year PFS rate of 43% vs. 5.4 months and 13%, respectively (HR =0.58; 97.5% CI: 0.41–0.81). Overall response rate in the high TMB population was 45.3% in the group treated with nivolumab plus ipilimumab and 26.9% in the group of patients receiving chemotherapy.
Among 363 patients with <1% PD-L1 expression, those treated with nivolumab plus chemotherapy experienced an improved PFS vs. patients receiving only chemotherapy (5.6 vs. 4.7 months, respectively; HR =0.74, 95% CI: 0.58–0.94) (14).
Moreover, these therapies are now being investigated also in the neoadjuvant and adjuvant settings. In these settings they may determine a significant benefit in survival. Newer combinations including pembrolizumab plus ipilimumab in pretreated NSCLC patients as well as durvalumab plus tremelimumab in the treatment-naïve population, are also being studied.
Therefore, the future for immunotherapy both in monotherapy and in combination with novel agents appears bright in lung cancer, also on the basis of the good tolerability profile, as reported by Brahmer et al. (7). Nevertheless, many unanswered questions about the most appropriate use of the treatment still remain, among the others: which is the optimal duration of therapy, which biomarkers will be predictive for response or toxicity, how acquired resistance to these agents occurs and which combinations can be the most effective in overcoming resistance.
Definitely significant progress will be made in the near future in addressing these questions.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837-46. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Brahmer JR, Rodríguez-Abreu D, Robinson AG, et al. Health-related quality-of-life results for pembrolizumab versus chemotherapy in advanced, PD-L1-positive NSCLC (KEYNOTE-024): a multicentre, international, randomised, open-label phase 3 trial. Lancet Oncol 2017;18:1600-9. [Crossref] [PubMed]
- Carbone DP, Reck M, Paz-Ares L, et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med 2017;376:2415-26. [Crossref] [PubMed]
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Paz-Ares L, Luft A, Tafreshi A, et al. Phase 3 study of carboplatin-paclitaxel/nab-paclitaxel (Chemo) with or without pembrolizumab (Pembro) for patients with metastatic squamous (Sq) non-small cell lung cancer (NSCLC). J Clin Oncol 2018;36:abstr 105.
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. [Crossref] [PubMed]
- Jotte RM, Cappuzzo F, Vynnychenko I, et al. IMpower131: Primary PFS and safety analysis of a randomized phase III study of atezolizumab + carboplatin + paclitaxel or nab-paclitaxel vs carboplatin + nab-paclitaxel as 1L therapy in advanced squamous NSCLC. J Clin Oncol 2018;36:abstr LBA9000.
- Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N Engl J Med 2018;378:2093-104. [Crossref] [PubMed]
- Borghaei H, Hellman MD, Paz-Ares LG, et al. Nivolumab (Nivo) + platinum-doublet chemotherapy (Chemo) vs chemo as first-line (1L) treatment (Tx) for advanced non-small cell lung cancer (NSCLC) with <1% tumor PD-L1 expression: Results from CheckMate 227. J Clin Oncol 2018;36:abstr 9001.


