Comparison of clinical efficacy between ultra-LABAs and ultra-LAMAs in COPD: a systemic review with meta-analysis of randomized controlled trials
Introduction
Chronic obstructive pulmonary disease (COPD) is a common respiratory disease characterized by largely irreversible airflow limitation (1). It is a major global health burden, and its prevalence is expected to increase, with an estimated annual death rate of over 4.5 million worldwide by 2030 (2). COPD patients often have chronic respiratory symptoms, exercise intolerance, and poor health-related quality of life, contributing to significant use of healthcare resources (1). In addition, a considerable proportion of COPD patients experience acute exacerbation and the frequent exacerbations are associated with rapid decline in lung function (3,4), aggravated quality of life (5,6), and increased mortality (7-10).
Long-acting bronchodilators are the cornerstone of the maintenance treatment in moderate to very severe COPD patients (1). Recently, numerous combinations of a long acting β2-agonist (LABA) and a long-acting muscarinic antagonist (LAMA) have been introduced in a single inhaler device, which showed consistently greater improvement in lung function than long acting bronchodilator monotherapy (11). However, single bronchodilators (LAMA or LABA) are still preferred as an initial treatment in a majority of COPD patients, and the combination of LABA/LAMA is recommended when patients have persistent symptoms despite the use of a single long-acting bronchodilator or experience frequent exacerbations (1). In addition, when patients do not obtain relief of symptoms with the addition of a second bronchodilator, step-down to a single bronchodilator is suggested. Among single long-acting bronchodilators, once-daily long-acting bronchodilators are preferred to twice-daily long-acting bronchodilators due to the advantage of covering a 24 h therapeutic window, which leads to improvements in drug adherence and treatment response (12-14).
Among long-acting bronchodilators, tiotropium is preferred for exacerbation prevention based on comparison to LABAs in two head-to-head comparisons (15,16). However, one of these two studies compared efficacy between tiotropium and salmeterol, a twice-daily LABA (15), and there is an absence of head-to-head comparative trials with newly introduced once-daily LAMA and LABA. Since there are no sufficient data supporting the use of once-daily LAMA (ultra-LAMA) over once-daily LABA (ultra-LABA) for exacerbation in COPD patients, we conducted a systemic review and meta-analysis for the comparisons of the clinical efficacy and safety of ultra-LABA (indacaterol, olodaterol, or vilanterol) and ultra-LAMA (tiotropium, glycopyrronium or umeclidinium) in stable patients with moderate to severe COPD.
Methods
Search strategy
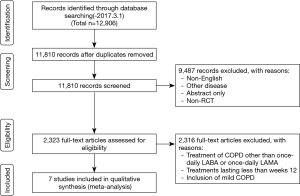
This systematic review is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Figure 1) (17). We identified published studies from MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials (up to March 1, 2017) databases using keywords related to COPD, LABAs, LAMAs, and randomized control trials (RCTs). The title and abstract were independently analyzed by two authors (E.Y.C and S.Y.K) for screening. Trials published solely in abstract form were excluded because the methods and results could not be fully analyzed. They independently assessed all studies for inclusion based on the criteria for study design, outcome, and intervention for participants. After they obtained full texts that could be potential candidates, they assessed and confirmed eligibility for the analysis. Disagreements were discussed and resolved by consensus.
Inclusion and exclusion criteria
The inclusion criteria for this study were as follows: (I) patients with moderate-to-severe COPD by Global Initiative for Chronic Obstructive Lung Disease (GOLD) diagnostic criteria; (II) randomized control trials (RCTs) with comparisons of ultra-LABAs (indacaterol, olodaterol, or vilanterol) and ultra-LAMAs (glycopyrronium, tiotropium, or umeclidinium); (III) at least 12 weeks of follow-up; and (IV) written in English. In this study, ultra-LABAs and ultra-LAMAs were defined as once-daily bronchodilators (18).
Data extraction
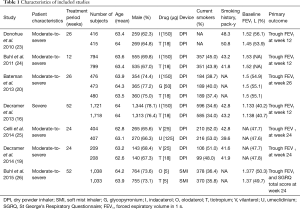
Data from the included studies were extracted and assessed for study characteristics and duration, doses of medications, inhaler devices, disease characteristics, age, sex, smoking history, trough forced expiratory volume in 1 second (FEV1), St. George’s Respiratory Questionnaire (SGRQ) total score, transition dyspnea index (TDI), and COPD worsening and exacerbation.
End points
The primary end point of this meta-analysis was the COPD exacerbation of ultra-LABAs versus ultra-LAMAs. COPD exacerbation was extracted from the reports of COPD worsening as the adverse events from six studies and in Decramer et al., the reports of COPD exacerbation with treatments was applied to COPD exacerbation (19). It is because Decramer et al. (19) reported only serious adverse events of exacerbation as adverse events. The secondary end points were change of trough FEV1 from baseline to the first follow-up after week 12 (≥ week 12), and St. SGRQ total score and TDI at the first follow-up after week 12 (≥ week 12). Since trough FEV1, SGRQ score, and TDI were measured at different time points according to the study protocol of each study, we used the data obtained at the first follow-up (week 12, week 24, or week 26) after week 12 from each study for the comparisons. The mean value of outcomes was used in the one study including two ultra-LAMAs (glycopyrronium and tiotropium) (20).
Risk of bias assessment
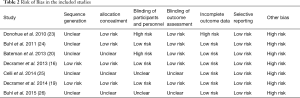
Two reviewers independently assessed the risk of bias of the included studies for sequence generation, allocation concealment, blinding of participants and researchers, blinding of outcome assessment, incomplete outcome data addressed, and free of selective reporting as recommended in the Cochrane Handbook of Systematic Reviews 5.1. (21).
Statistical analysis
Statistical analysis was performed using the R 3.3.2 (Vienna, Austria; http://www.R-project.org/) package ‘metafor’ for meta-analysis of the included studies. Mean differences (MDs) or odds ratios (ORs) for end points were estimated and tested under the fixed effect model or the random effect model. The pooled data were applied to a random effect model if heterogeneity was present in the data of more than 4 studies and to a fixed effect model otherwise. The precision of the estimates was quantified by the 95% confidence interval (CI). Heterogeneity was measured using the Higgins and Green I2 test, which is calculated as 100%×(Q–df)/Q, where Q is the observed chi-square statistic, and the degrees of freedom (df) is the number of studies less one. The value of I2 ranges between 0% (no heterogeneity) and 100% (maximal heterogeneity), and heterogeneity was considered to be mild (<30%), moderate (30–60%), and high (>60%) (21). We also evaluated potential publication bias using Egger’s regression test and the funnel-plot (22). P values <0.05 (two-tailed test) were considered significant.
Results
Study characteristics
Figure 1 shows how the relevant studies were identified. A total of 12,906 articles were found by searching databases. Results obtained from 10,591 patients with COPD [LABA (n=5,058); LAMA (n=5,533)] were selected from 7 published papers. Three studies reported on indacaterol vs. tiotropium (16,23,24) and one study on indacaterol vs. tiotropium and indacaterol vs. glycopyrronium (20). There were one study with vilanterol vs. umeclidinium (25), one study with vilanterol vs. tiotropium (19), and one study with olodaterol vs. tiotropium (26).
All studies published by March 2017 were included, and relevant patient baseline characteristics are summarized in Table 1. All RCTs were randomized and double-blind, and we included comparisons with open-label tiotropium in the RCTs. The duration of follow-up ranged from 12 to 56 weeks.
Full table
Primary outcome
As shown in Figure 2A, the analysis of 7 studies comparing ultra-LABA with ultra-LAMA showed that ultra-LAMA resulted in less frequent COPD exacerbations than ultra-LABA (OR =0.857; 95% CI, 0.783–0.938; P=0.0008). When the one paper with only severe COPD was excluded in the meta-analysis (16), COPD exacerbation also occurred less frequently with ultra-LAMA than ultra-LABA (OR =0.885; 95% CI, 0.784–0.998; P=0.0471) (Figure 2B).

Secondary outcomes
Change of trough FEV1 from baseline
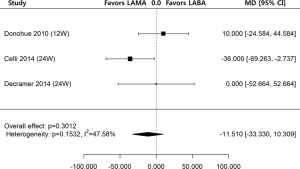
Regarding change of trough FEV1 from baseline to the first follow-up after week 12 (≥ week 12), the data from 3 out of 7 studies were available for the means and standard errors of changes. The one study reported results at week 12 (23), and the remaining two studies reported results at week 24 (19,25). The analysis of three studies of ultra-LABA and ultra-LAMA showed no significant differences in the change of trough FEV1 at either week 12 or 24 from baseline (MD =−11.510 mL; 95% CI, −33.330–10.309; P=0.3012) (Figure 3).
SGRQ score and TDI changes
At the first follow-up, SGRQ total score was reported in six studies; the exception was one study in which only the change in SGRQ score was available (Figure 4A) (19). The SGRQ total scores at week 12 were reported in 3 studies (16,23,24), at week 24 in 2 studies (25,26), and at week 26 in the remaining studies (20). In these six studies, the MD was −0.621, which was not significant (95% CI, −1.603 to 0.362; P=0.2159).

At the first follow-up, TDI was reported in all 7 studies. The TDI at week 12 was reported in 3 studies (16,23,24), at week 24 in 3 studies (19,25,26), and at week 26 (20) in the remaining one study (Figure 4B). As shown in Figure 4B, there was no significant difference in TDI at the first follow-up between the ultra-LABA and ultra-LAMA groups (MD =0.054; 95% CI, −0.197 to 0.305; P=0.6746).
Heterogeneity and Bias in the included studies
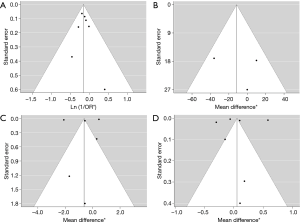
High levels of heterogeneity were detected on SGRQ total score change (I2=99.82%, P<0.0001) and TDI change (I2=99.82%, P<0.0001). There was moderate level of heterogeneity on change of trough FEV1 from baseline (I2=47.58%, P=0.1532) and mild level of heterogeneity on COPD exacerbation (I2=0.00%, P=0.7619). The assessments performed by the authors of each risk of bias item for each included RCT are summarized in Table 2. All papers were funded by pharmaceutical companies. Two studies were at high risk for blinding of participants and personnel because they used open-labeled tiotropium (20,23). For publication bias, significant asymmetry in the funnel-plot was not detected for the primary and secondary outcomes (P=0.6630 for COPD exacerbation, P=0.7898 for change of trough FEV1 from baseline, P=0.7078 for SGRQ total score change, and P=0.7495 for TDI change) (Figure 5). Therefore, we assumed there was no evidence of publication bias.
Full table
Discussion
In this study, we performed a meta-analysis on the clinical efficacy and safety of ultra-LABA (indacaterol, olodaterol, or vilanterol) versus ultra-LAMA (glycopyrronium, tiotropium, or umeclidinium) in stable patients with moderate-to-severe COPD. The main finding of this meta-analysis was that COPD exacerbation occurred less often with the ultra-LAMAs than ultra-LABAs among patients with moderate-to-severe COPD, while ultra-LAMAs were as effective as ultra-LABAs in terms of trough FEV1 and quality of life including SGRQ and TDI at the first follow-up.
A previous meta-analysis based on four studies [Donohue et al. (23), Buhl et al. (24), Bateman et al. (20), and Decramer et al. (16)] comparing the clinical efficacy and safety of tiotropium, a ultra-LAMA, and indacaterol, a ultra-LABA, showed that COPD worsening was more frequent in the indacaterol group than in the tiotropium group (27). We affirmed these findings with various types of ultra-LABAs and ultra-LAMAs and a larger number of studies. Our findings also support the current recommendations of the GOLD guideline (1), which reports an advantage of ultra-LAMAs over ultra-LABAs in exacerbation-prevention properties. The potential mechanisms might be explained by the anti-inflammatory effects of LAMA (28), which was shown in an animal model of COPD (e.g., reduction of neutrophils and proinflammatory cytokine levels and inhibition of mucus secretion as well as airway remodeling) (29-31). However, the results of our study need careful interpretation. First, we used COPD worsening in the adverse events for the analysis of COPD exacerbation except for Decramer et al. (19) as COPD worsening has comprehensive meaning including exacerbation. This is because only one head-to-head comparative study between tiotropium and indacaterol for COPD exacerbation has been published. Secondly, the number of studies including ultra-LAMAs or ultra-LABAs other than tiotropium or indacaterol was relatively small. In addition, since a considerable number of studies in our study included tiotropium, it might not be generalized to all ultra-LAMAs. When in vitro pharmacological profiles were observed using human bronchus, the potency, onset of action, and offset of action were different among LAMAs (32). In addition, as most studies on the anti-inflammatory effects of LAMAs were performed with tiotropium (33,34), further studies are needed to elucidate whether other ultra-LAMAs exhibit similar features to tiotropium (31).
With respect to lung function, our study showed results with the change of trough FEV1 from baseline, which was consistent with the previous meta-analysis showing no significant differences of trough FEV1 at week 12 between the indacaterol and tiotropium groups (27). Ultra-LAMAs also showed a similar effect to ultra-LABAs with respect to quality of life measured by SGRQ total score and TDI in the analyses including data at 12 weeks (16,23,24), 24 weeks (19,25,26), or 26 weeks (20). However, in the subgroup analysis, compared to ultra-LAMAs, ultra-LABAs significantly improved SGRQ total score and TDI at 12 weeks (Figure S1A,B). The significant differences in SGRQ total score and TDI at 12 weeks between ultra-LAMAs and ultra-LABAs might suggest a faster improvement of quality of life in ultra-LABAs than ultra-LAMAs despite significant heterogeneities in the analyses.

Our study has an advantage that our analyses were relatively free of publication bias in funnel plots, which enhances the reliability of the results of the meta-analyses. Although high levels of heterogeneity were detected in SGRQ total score change and TDI change, there was only mild heterogeneity in COPD exacerbation. Thus, the exacerbation prevention effect of ultra-LAMA over ultra-LABA, the primary outcome in this study, can be considered to be based on high quality of meta-analyses, which is a major strength of this study.
In summary, this study suggests that COPD exacerbation occurred less often with ultra-LAMAs than ultra-LABAs with no significant differences in improvement of lung function or quality of life in moderate-to-severe COPD patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: CK Rhee has received consulting/lecture fees from MSD, AstraZeneca, Novartis, GSK, Takeda, Mundipharma, Sandoz, Boehringer-Ingelheim, and Teva-Handok. HY Park has received lecture fees from AstraZeneca, Novartis, and Boehringer-Ingelheim. The other authors have no conflicts of interest to declare.
References
- GOLD. Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2018. Available online: http://www.goldcopd.org/. Accessed April 4, 2018.
- Lopez AD, Shibuya K, Rao C, et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J 2006;27:397-412. [Crossref] [PubMed]
- Donaldson GC, Seemungal TA, Bhowmik A, et al. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002;57:847-52. [Crossref] [PubMed]
- Kanner RE, Anthonisen NR, Connett JE. Lower respiratory illnesses promote FEV(1) decline in current smokers but not ex-smokers with mild chronic obstructive pulmonary disease: results from the lung health study. Am J Respir Crit Care Med 2001;164:358-64. [Crossref] [PubMed]
- Kessler R, Stahl E, Vogelmeier C, et al. Patient understanding, detection, and experience of COPD exacerbations: an observational, interview-based study. Chest 2006;130:133-42. [Crossref] [PubMed]
- Spencer S, Calverley PM, Burge PS, et al. Impact of preventing exacerbations on deterioration of health status in COPD. Eur Respir J 2004;23:698-702. [Crossref] [PubMed]
- Piquet J, Chavaillon JM, David P, et al. High-risk patients following hospitalisation for an acute exacerbation of COPD. Eur Respir J 2013;42:946-55. [Crossref] [PubMed]
- Singanayagam A, Schembri S, Chalmers JD. Predictors of mortality in hospitalized adults with acute exacerbation of chronic obstructive pulmonary disease. Ann Am Thorac Soc 2013;10:81-9. [Crossref] [PubMed]
- Gunen H, Hacievliyagil SS, Kosar F, et al. Factors affecting survival of hospitalised patients with COPD. Eur Respir J 2005;26:234-41. [Crossref] [PubMed]
- Soler-Cataluña JJ, Martinez-Garcia MA, Roman Sanchez P, et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005;60:925-31. [Crossref] [PubMed]
- Farne HA, Cates CJ. Long-acting beta2-agonist in addition to tiotropium versus either tiotropium or long-acting beta2-agonist alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2015.CD008989. [PubMed]
- Kornmann O, Dahl R, Centanni S, et al. Once-daily indacaterol versus twice-daily salmeterol for COPD: a placebo-controlled comparison. Eur Respir J 2011;37:273-9. [Crossref] [PubMed]
- Korn S, Kerwin E, Atis S, et al. Indacaterol once-daily provides superior efficacy to salmeterol twice-daily in COPD: a 12-week study. Respir Med 2011;105:719-26. [Crossref] [PubMed]
- Dahl R, Chung KF, Buhl R, et al. Efficacy of a new once-daily long-acting inhaled beta2-agonist indacaterol versus twice-daily formoterol in COPD. Thorax 2010;65:473-9. [Crossref] [PubMed]
- Vogelmeier C, Hederer B, Glaab T, et al. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med 2011;364:1093-103. [Crossref] [PubMed]
- Decramer ML, Chapman KR, Dahl R, et al. Once-daily indacaterol versus tiotropium for patients with severe chronic obstructive pulmonary disease (INVIGORATE): A randomised, blinded, parallel-group study. Lancet Respir Med 2013;1:524-33. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [Crossref] [PubMed]
- Domingo C. Ultra-LAMA, ultra-LABA, ultra-inhaled steroids? The future has landed. Arch Bronconeumol 2013;49:131-4. [PubMed]
- Decramer M, Anzueto A, Kerwin E, et al. Efficacy and safety of umeclidinium plus vilanterol versus tiotropium, vilanterol, or umeclidinium monotherapies over 24 weeks in patients with chronic obstructive pulmonary disease: results from two multicentre, blinded, randomised controlled trials. Lancet Respir Med 2014;2:472-86. [Crossref] [PubMed]
- Bateman ED, Ferguson GT, Barnes N, et al. Dual bronchodilation with QVA149 versus single bronchodilator therapy: The SHINE study. Eur Respir J 2013;42:1484-94. [Crossref] [PubMed]
- Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [Crossref] [PubMed]
- Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [Crossref] [PubMed]
- Donohue JF, Fogarty C, Lotvall J, et al. Once-daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropium. Am J Respir Crit Care Med 2010;182:155-62. [Crossref] [PubMed]
- Buhl R, Dunn LJ, Disdier C, et al. Blinded 12-week comparison of once-daily indacaterol and tiotropium in COPD. Eur Respir J 2011;38:797-803. [Crossref] [PubMed]
- Celli B, Crater G, Kilbride S, et al. Once-daily umeclidinium/vilanterol 125/25 mcg in COPD: a randomized, controlled study. Chest 2014;145:981-91. [Crossref] [PubMed]
- Buhl R, Maltais F, Abrahams R, et al. Tiotropium and olodaterol fixed-dose combination versus mono-components in COPD (GOLD 2-4). Eur Respir J 2015;45:969-79. [Crossref] [PubMed]
- Kim JS, Park J, Lim SY, et al. Comparison of clinical efficacy and safety between indacaterol and tiotropium in COPD: Meta-analysis of randomized controlled trials. PLoS One 2015;10:e0119948. [Crossref] [PubMed]
- Kistemaker LE, Gosens R. Acetylcholine beyond bronchoconstriction: roles in inflammation and remodeling. Trends Pharmacol Sci 2015;36:164-71. [Crossref] [PubMed]
- Wollin L, Pieper MP. Tiotropium bromide exerts anti-inflammatory activity in a cigarette smoke mouse model of COPD. Pulm Pharmacol Ther 2010;23:345-54. [Crossref] [PubMed]
- Pera T, Zuidhof A, Valadas J, et al. Tiotropium inhibits pulmonary inflammation and remodelling in a guinea pig model of COPD. Eur Respir J 2011;38:789-96. [Crossref] [PubMed]
- Alagha K, Palot A, Sofalvi T, et al. Long-acting muscarinic receptor antagonists for the treatment of chronic airway diseases. Ther Adv Chronic Dis 2014;5:85-98. [Crossref] [PubMed]
- Naline E, Grassin Delyle S, Salvator H, et al. Comparison of the in vitro pharmacological profiles of long-acting muscarinic antagonists in human bronchus. Pulm Pharmacol Ther 2018;49:46-53. [Crossref] [PubMed]
- Matthiesen S, Bahulayan A, Kempkens S, et al. Muscarinic receptors mediate stimulation of human lung fibroblast proliferation. Am J Respir Cell Mol Biol 2006;35:621-7. [Crossref] [PubMed]
- Bühling F, Lieder N, Kuhlmann UC, et al. Tiotropium suppresses acetylcholine-induced release of chemotactic mediators in vitro. Respir Med 2007;101:2386-94. [Crossref] [PubMed]