The impact on mediastinal recurrence based on the number of harvested mediastinal lymph nodes and assessed N2 Stations in patients with stage I invasive lung adenocarcinoma
Introduction
One of the most common malignancies, lung cancer, is the leading cause of cancer-related mortality and morbidity in the world, with almost 85% of cases being non-small cell lung cancer (NSCLC) (1,2). Patients with clinical stage IA NSCLC constituted as high as 36% of total lung cancer patients according to the International Association for the Study of Lung Cancer (IASLC) (3). Although stage I NSCLC has significantly a better survival outcome than that of advanced stage NSCLC there are still 30% of patients suffering from postoperative recurrence (including local recurrence and distant metastasis) when receiving a complete resection (4,5). Although a distant metastasis is a major determinant of secondary survival, an intrathoracic locoregional recurrence is a common postoperative failure pattern. One of the explanations for this is the potential difference between MLD dissection and N2 station assessment among patients with pathological stage I IADC.
Unfortunately, guidelines and recommendations for clinicians to identify optimal MLN dissection are still lacking. Some studies have shown that MLN dissection significantly aided in the prognosis and staging (6-9), while other surgeons have asserted it is only useful for cancer staging but not prognosis (10). Still other researchers have even declared that MLN dissection could potentially increase longer operative time and postoperative surgery-related morbidity (11-14). Each of these conflicting viewpoints has made surgeons routine practice varies from mere visual inspection of the unopened mediastinum to radical lymphadenectomy (15).
The lymphatic drainages from primary tumors to the hilar and MLNs have been investigated for over 60 years (16). MLN dissection has been of great significance for prognosis of potential postoperative mediastinal recurrence among those early-staged IADC. Hence, we aimed to evaluate the association between the number of MLNs and N2 stations harvested and the MS-RFS rate based on a study of 2,199 patients with pathologic stage I IADC from the Shanghai Chest Hospital.
Methods
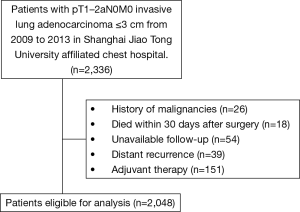
The institutional review board of the Shanghai Chest Hospital supported this study and provided informed consent for our operation. Patients with stage I IADC who underwent curative resection between 2009 and 2013 were all identified for potential inclusion. Patients with a history of malignancy within 5 years (including metastatic tumors in lung), small cell lung cancer and distant metastases were ineligible for the analysis. We also excluded patients who received induction treatments, and/or whose follow-up information could not be collected. Additionally, patients receiving adjuvant therapy were excluded from the series. Atypical adenomatous hyperplasia (AAH), adenocarcinoma in situ (AIS) and minimally invasive adenocarcinoma (MIA) are non-invasive lesions and free from LN metastasis. Thus, patients with these lesions were also excluded. A summary of the patient selection process is shown in Figure 1. All the patients received preoperative assessments including chest CT scanning, abdominal CT scan or ultrasound, brain CT scanning or MRI and radionuclide bone scanning, to exclude distant metastases. Positron emission tomography (PET)-CT was an optimal substitution if necessary.
The resected lung specimens and LNs were fixed in 10% formalin and then embedded in paraffin. Subsequently, the hematoxylin and eosin (HE)-stained sections were evaluated microscopically by two experienced pulmonary pathologists (Jie Zhang, Yuchen Han).
The pathological subtypes of resected stage I ADC were classified into 5 major histological patterns including lepidic, acinar, papillary, solid, and micropapillary predominant subtypes, along with their related variants. Classification was completed according to the recommendations published in 2011 (17) by the joint effort of the International Association for the Study of Lung Cancer (IASLC), the American Thoracic Society (ATS), and the European Respiratory Society (ERS), which recorded in 5% increment for adenocarcinomas. In this novel proposal, different kinds of subtypes were related with different prognoses, with lepidic (L) labeled as favorable, acinar and papillary (A+P) labeled as intermediate, and micropapillary and solid (M+S) labeled as poor.
The number of MLNs was obtained by reviewing operative notes and pathology reports. Lymph nodes were named according to the American Thoracic Society’s (ATS) lymph node stations (18). LN stations from the 1st to the 9th were assigned under the N2 station label. The terms used by the surgeons in the operative reports to qualify the technique of lymph node evaluation were not taken into consideration. If more than one sample was obtained from a single station during surgery, it was counted as a single lymph node. Mediastinal recurrence was defined according to the CT and PET-CT findings. In a CT scan, a mediastinal lymph node (MLN) with a short axis diameter measuring 10 mm or more was determined to indicate clinical N2 recurrence. On PET-CT scan, a SUVmax value greater than 2.5 was diagnosed as a PET-positive lymph node.
After the exclusions, a total of 2,048 patients met the standards. All patients were first followed up through out-patient clinic or telephone every 3 months for the first year after surgery, every 6 months for the next 3 years, and then annually after that.
Statistical analysis
All the clinicopathologic data and distributions of survival were analyzed by SPSS 23.0 software package (SPSS Inc., Chicago, IL, USA) and Prism 5 (GraphPad Software Inc., La Jolla, CA, USA). The curves of RFS, as well as the comparisons, were calculated by Kaplan-Meier survival curves and the log-rank test. Two-sided P<0.05 was set as statistical significance in this study.
Results
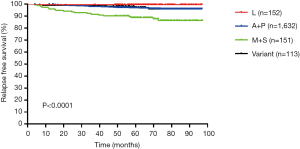
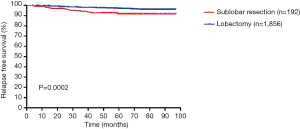
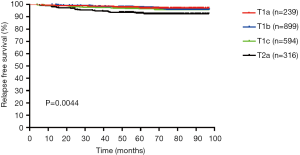
The clinical and pathological characteristics of our primary cohort are listed in Table 1. Of the 2,048 patients included in the study, there were 1,215 males (59.3%) and 833 females (40.7%), with an average age of 60.3 years (28–85 years). Lobectomy accounted for the majority of surgical treatment (90.6%). Furthermore, the tumor on the right side (63.3%) was redundant against the left side (36.7%). The 3- and 5-year MS-RFS rate was 97.8% and 93.8%, respectively. Unsurprisingly, the SOL/MIP pathological subtypes, sublobectomy and T2a stage definitively correlated with the worse MS-RFS (Figures S1-S3).
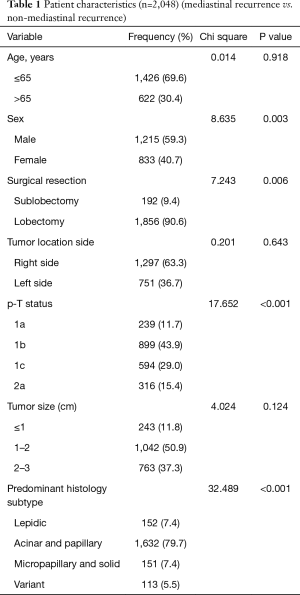
Full table
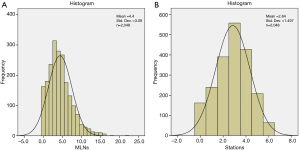
The distribution of MLNs and N2 stations all conformed to normal distribution (Figure 2A,B), the mean number of total MLNs and N2 stations was 4.40±3.09 (0–22 nodes) and 2.84±1.497 (0–6 stations), respectively. We averaged 1.55 of the number of lymph nodes obtained from each station. We took the number of 5 lymph nodes and 3 N2 stations as the cutoff value in this research. The details in the lymph node assessment are listed in Table 2. The Kaplan-Meier survival curves for MS-RFS are depicted in Figures 3,4.
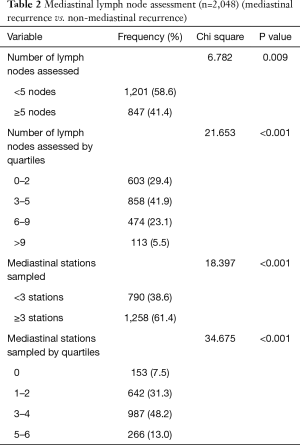
Full table
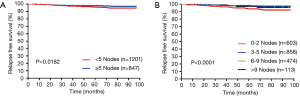
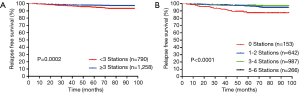
MS-RFS curves depending on number of harvested MLNs and assessed N2 stations
For patients with ≥5 MLNs, mediastinum-specific relapse-free survival (MS-RFS) rate were 98.3% and 96.6% for 3- and 5-year, respectively, which significantly demonstrated better survival outcome against those <5 (96.3% and 92.8%, respectively, Log-rank P=0.018). We then divided the patients into 4 groups on the basis of the total number of MLNs (0 to 2, 3 to 5, 6 to 9, and more than 9 lymph nodes), and found that the 3- and 5-year MS-RFS rates were 96.4%, 98.1%, 98.8%, 99.4% and 91.3%, 95.8%, 96.4%, 93.5% (P<0.001), respectively. The number of assessed N2 stations was also calculated for patients with assessed N2 stations <3, and the MS-RFS was found to be 95.5% and 90.3% at 3- and 5-year, respectively, while the patients with assessed stations ≥3 showed results of 98.2% and 95.8% of MS-RFS at 3- and 5-year, respectively (P<0.001). Stations divided into 4 groups (0, 1 to 2, 3 to 4 and more than 4) also showed more improvement in the MS-RFS rate as the number of assessed N2 stations increased (P<0.001).
Univariable analysis indicated that sex, T stage, tumor size, surgical resection, predominant histology subtype, number of harvested MLNs, and assessed N2 stations were all potential survival predictors of MS-RFS. Moreover, T stage, surgical resection, predominant histology subtype and number of N2 stations assessed were still independently significant factors of MS-RFS in multivariable analysis, whereas sex, tumor size, and number of MLNs assessed were not (Table 3).
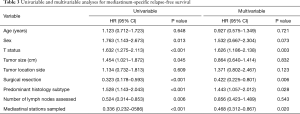
Full table
Discussion
Opinions still vary among surgeons as to whether to remove all, some, or none of the MLNs during surgical resection; therefore, we sought to determine the number of harvested MLNs and assessed N2 stations that should be considered for the accurate prediction of prognosis in pathologic stage I IADC.
This retrospective analysis of our data revealed that the number of assessed N2 stations was a significant predictor of MS-RFS in multivariable analysis for patients with stage I IADC; an improved MS-RFS rate was obtained the more N2 stations were resected. Gajra et al. (15) also observed that, in patients with stage I NSCLC, a greater number of N2 stations studied had an improved RFS compared with patients with fewer N2 stations examined (P<0.001). Given the subjectivity and variability in MLNs resection, the clinical significance of the MS-RFS differences between MLNs groups is still questionable. It is worth remembering that the quality of the complete mediastinal lymphadenectomy depends on its anatomical boundaries rather than on the number of resected MLNs (19,20). Our study demonstrated that the number of MLNs and stations assessed were not significant predictors of the overall survival (OS) in multivariable analysis (data not shown) (HR =0.623; 95% CI, 0.312–1.327, P=0.173 and HR =1.456; 95% CI, 0.703–2.958, P=0.324, respectively), which means that the extent of MLN dissection is not related to the patient’s long-term survival rate. Several studies have presented similar results. For instance, Darling et al. (21) reported in a prospective randomized controlled clinical trial (ACOSOG Z0030) that there was no difference in long-term survival between systemic MLN dissection and MLN sampling during the resection for patients with T1 or T2, N0 or non-hilar N1 NSCLC. The median survival for MLNS is 8.1 years, and it is 8.5 years for MLN dissection (P=0.250). However, all subjects in his study included patients with tumor from stage IA to IIIB, and the pathological types of their research included squamous cell, adenocarcinoma, large cell, bronchoalveolar, and other NSCLC. Moreover, their extent of resection varied from R0 to R2 while our surgery was all R0 resection. These incongruities may partly explain the difference in results. Sugi et al. (22) also found that systematic MLN dissection showed no superiority against MLN sampling statistically in the 5-year survival rate among T1N0M0 NSCLC. The results reveal that the extent of MLN dissection is not related to OS, although we still strongly recommend clinicians to perform a standard systematic mediastinal lymphadenectomy for a lower mediastinal recurrence and a better tumor staging.
With regards to the predominant histological subtypes, M+S plotted a worse recurrence rate on the MS-RFS curves when compared with subgroups of L and A+P. Cha and associates (23) reviewed 511 patients with lung adenocarcinoma ≤3 cm, and found that the patients with lepidic predominant tumors had a good prognosis but those with solid and micropapillary predominant tumors presented an opposite trend. Their results are essentially in line with what we discovered and in compliance with the pathological classification published by the IASLC/ATS/ERS in 2011 (24).
The present meta-analysis revealed that the stage I lung cancer patients undergoing sublobectomy after intentional selection achieved comparable survival to those who received lobectomy (25). As for each of the 2,048 IADC patients who underwent lobectomy, they had a better MS-RFS rate than those who underwent sublobectomy (P<0.001). These results also agree with the NCCN guidelines for MIA surgery requirements that strongly recommend lobectomy combined with systematic lymph node dissection or sampling for stage I IADCs.
There were several limitations in this study. First, our study didn’t elaborate which certain kind of N2 stations were included in our assessed N2 station. In other word, it was the number not the certain station that made sense, so this hypothesis and results still needed further investigation. Second, those clinical and pathological data were retrospective in nature, and they should be confirmed in prospective trials if possible.
In summary, our finding demonstrated that T status, surgical resection, predominant histology subtype and assessed N2 stations were independently significant indicators of MS-RFS. In order to improve MS-RFS among patients with pathological stage I IADC, we recommend the cutoff values for harvested MLNs and assessed N2 stations be 5 and 3, respectively,.
Acknowledgements
Funding: This work was supported by the National Natural Science Foundation of China (81773007); Shanghai Municipality: Shanghai Rising-Star Program (16QA1403500); Funding for Shanghai Fostering Talents by Shanghai Municipal Human Resources and Social Security Bureau (201706); Outstanding Youth Program of the Shanghai Municipal Commission of Health and Family Planning (2017YQ018).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Review Board [No. KS (P1733)] and written informed consent was obtained from all patients.
References
- Tanaka F, Yoneda K. Adjuvant therapy following surgery in non-small cell lung cancer (NSCLC). Surg Today 2016;46:25-37. [Crossref] [PubMed]
- Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 2006;24:2137-50. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Doddoli C, Aragon A, Barlesi F, et al. Does the extent of lymph node dissection influence outcome in patients with stage I non-small-cell lung cancer? Eur J Cardiothorac Surg 2005;27:680-5. [Crossref] [PubMed]
- Mountain CF. Revisions in the international system for staging lung cancer. Chest 1997;111:1710-7. [Crossref] [PubMed]
- Naruke T. Significance of lymph node metastases in lung cancer. Semin Thorac Cardiovasc Surg 1993;5:210-8. [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Ann Thorac Surg 1995;60:615-22. [Crossref] [PubMed]
- Nwogu CE, Groman A, Fahey D, et al. Number of lymph nodes and metastatic lymph node ratio are associated with survival in lung cancer. Ann Thorac Surg 2012;93:1614-9. [Crossref] [PubMed]
- Osarogiagbon RU, Ogbata O, Yu X. Number of lymph nodes associated with maximal reduction of long-term mortality risk in pathologic node-negative non–small cell lung cancer. Ann Thorac Surg 2014;97:385-93. [Crossref] [PubMed]
- Saji H, Tsuboi M, Yoshida K, et al. Prognostic impact of number of resected and involved lymph nodes at complete resection on survival in non-small cell lung cancer. J Thorac Oncol 2011;6:1865-71. [Crossref] [PubMed]
- Izbicki JR, Thetter O, Habekost M, et al. Radical systematic mediastinal lymphadenectomy in non‐small cell lung cancer: a randomized controlled trial. Br J Surg 1994;81:229-35. [Crossref] [PubMed]
- Izbicki JR, Passlick B, Pantel K, et al. Effectiveness of radical systematic mediastinal lymphadenectomy in patients with resectable non-small cell lung cancer: results of a prospective randomized trial. Ann Surg 1998;227:138-44. [Crossref] [PubMed]
- Lardinois D, Suter H, Hakki H, et al. Morbidity, survival, and site of recurrence after mediastinal lymph-node dissection versus systematic sampling after complete resection for non-small cell lung cancer. Ann Thorac Surg 2005;80:268-74. [Crossref] [PubMed]
- Okada M, Sakamoto T, Yuki T, et al. Selective mediastinal lymphadenectomy for clinico-surgical stage I non–small cell lung cancer. Ann Thorac Surg 2006;81:1028-32. [Crossref] [PubMed]
- Gajra A, Newman N, Gamble G P, et al. Effect of number of lymph nodes sampled on outcome in patients with stage I non–small-cell lung cancer. J Clin Oncol 2003;21:1029-34. [Crossref] [PubMed]
- Nohl HC. An investigation into the lymphatic and vascular spread of carcinoma of the bronchus. Thorax 1956;11:172-85. [Crossref] [PubMed]
- Travis W D, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest 1997;111:1718-23. [Crossref] [PubMed]
- Martini N, Flehinger BJ, Zaman MB, et al. Results of resection in non-oat cell carcinoma of the lung with mediastinal lymph node metastases. Ann Surg 1983;198:386-97. [Crossref] [PubMed]
- Legras A, Mordant P, Arame A, et al. Long-term survival of patients with pN2 lung cancer according to the pattern of lymphatic spread. Ann Thorac Surg 2014;97:1156-62. [Crossref] [PubMed]
- Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non–small cell carcinoma: Results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011;141:662-70. [Crossref] [PubMed]
- Sugi K, Nawata K, Fujita N, et al. Systematic lymph node dissection for clinically diagnosed peripheral non-small-cell lung cancer less than 2 cm in diameter. World J Surg 1998;22:290-4. [Crossref] [PubMed]
- Cha MJ, Lee HY, Lee KS, et al. Micropapillary and solid subtypes of invasive lung adenocarcinoma: clinical predictors of histopathology and outcome. J Thorac Cardiovasc Surg 2014;147:921-928.e2. [Crossref] [PubMed]
- Travis W D, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/American thoracic society/European respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Zhang Y, Sun Y, Wang R, et al. Meta‐analysis of lobectomy, segmentectomy, and wedge resection for stage I non‐small cell lung cancer. J Surg Oncol 2015;111:334-40. [Crossref] [PubMed]