Application of standardized hemodynamic protocols within enhanced recovery after surgery programs to improve outcomes associated with anastomotic leak and conduit necrosis in patients undergoing esophagectomy
Introduction
Esophagectomy remains an important component of the treatment of localized esophageal and gastroesophageal junctional cancer in physiologically appropriate patients (1). The operation is associated with a high risk for postoperative complications, which affect morbidity, mortality, cost, and negatively impact upon long-term health related quality of life (2-4). Hemodynamic deregulation that occurs commonly in the setting of esophagectomy may predispose patients to morbidity through impaired tissue perfusion.
The introduction of enhanced recovery after surgery (ERAS) pathways and centralization of care to high volume centers has been shown to reduce postoperative morbidity (5-8). The focus of perioperative management should be to optimize the patient’s physiology in order to facilitate postoperative recovery. ERAS guidelines provide a framework within which therapeutic goals can be set and clear strategies enacted where there is the potential for unintended deviation from the expected course. Several central components of ERAS pathways including restrictive fluid protocols, epidural analgesia, and early mobilization, while intended to advance recovery may also contribute to perioperative hypotension. A balanced approach to perioperative care should include adherence to ERAS principals (7) whilst maintaining appropriate hemodynamic conditions for perfusion of organs and the gastric conduit.
The Esophageal Complications Consensus Group (ECCG) has reported a 59% overall complication rate, 11.4% anastomotic leak, and 1.3% incidence of conduit necrosis in a large contemporary international multicenter study including only high-volume centers (9). While reported rates of anastomotic leak after esophagectomy vary widely between individual centers (4–25%), it remains the most commonly encountered major postoperative complication (10-15). Likewise, conduit necrosis, which is caused by impaired perfusion and subsequent ischemia of the proximal part of the conduit, is a devastating postoperative complication frequently requiring immediate re-operation, often in the setting of multiple organ failure and high postoperative mortality (4). For those surviving conduit necrosis there still remains the ominous challenge of reconstruction. The tenuous blood supply to the conduit, mainly based on a single feeding vessel, makes this organ particularly vulnerable to ischemia. The administration of neoadjuvant chemoradiotherapy can also affect the microvascular perfusion of the proximal stomach (16-18). Establishing and maintaining good perfusion to the conduit reduces the risk of anastomotic leak and conduit necrosis (19,20).
The aim of this review is to provide a systematic review of the current literature concerning hemodynamic protocols in the perioperative management of esophageal and gastroesophageal junctional cancer and to present a standardized hemodynamic protocol (SHP) for use in the immediate postoperative period after esophagectomy to decrease the incidence of anastomotic leak and conduit necrosis.
Methods
We conducted a literature search to identify relevant studies in PubMed and the Web of Science. Randomized controlled trials (RCTs), high quality meta analyses, and retrospective studies published between 1988 and 2018 were identified using the search terms: “hemodynamic monitoring” or “fluid restriction” or “goal-directed treatment” and “esophagectomy” or “esophagogastrectomy” or “esophageal surgery”. All articles were written in English. Inclusion criteria were studies reporting on esophagectomy patients and hemodynamic protocols in some form. Exclusion criteria were studies including non-esophageal surgery.
An SHP was introduced in 2004 at Virginia Mason Medical Center; it was developed by a multidisciplinary team of surgeons, anesthesiologists, intensivists, nursing staff and physiotherapists. The prospective IRB approved esophagectomy database at Virginia Mason Medical Center was used to identify the incidence of anastomotic leak and conduit necrosis since the initiation of the SHP.
Literature review of hemodynamic protocols for esophagectomy
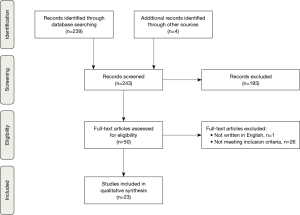
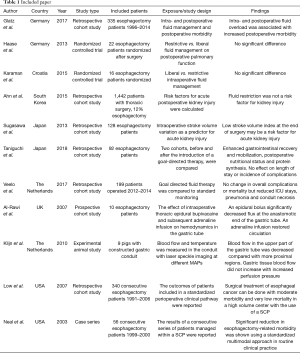
Literature search resulted in 243 articles. One article was excluded, as it was not written in the English language. After a review of the titles and abstracts, 50 articles were chosen for full text review, of these 23 articles met the inclusion criteria (Figure 1). The included articles are presented in Table 1 (21-43).
Full table
Perioperative hemodynamic management in esophageal surgery has been an area of interest for many years but there are few high quality studies and little scientific evidence regarding SHPs (37). A number of retrospective studies have found that a positive cumulative fluid balance after esophagectomy was associated with increased length of stay, increased risk for postoperative pulmonary and cardiac complications (24,29,41).
Methods of assessing hemodynamic status
The hemodynamic status of a patient is multifactorial and difficult to evaluate with high certainty. As such there are different strategies to evaluate the hemodynamic status in the perioperative period (44). Arterial pressure variation guided fluid management is a technique that uses the peripheral arterial catheter to calculate variation in stroke volume and a stroke volume index. The technique can be used to evaluate fluid responsiveness during surgery and in the postoperative period (26,45). Fluid management that was guided by stroke volume variation after esophagectomy was evaluated in two small studies and was shown to be a reliable predictor for intravascular volume depletion and possible hypotension in the postoperative period (31,32).
One study of ten patients undergoing esophagectomy showed that central venous pressure was not a reliable predictor of intravascular volume in the postoperative period compared to pressure parameters including the diameter of the inferior vena cava and the left ventricle (35). Anesthetic management, the position of the patient, and the surgical technique during esophagectomy also impacts upon hemodynamic status during surgery (33,42,43).
Restrictive fluid therapy
A restricted fluid strategy with intraoperative fluid limited to <4 liters and mean arterial blood pressure (MAP) maintained above 65 mmHg with the use of norepinephrine infusion in combination with early postoperative extubation has been shown to significantly reduce pneumonia in a cohort of 83 patients who underwent open esophagectomy (23). Fluid balance above average on the day of surgery, and the first 4 postoperative days was associated with increased risk for postoperative complications in another retrospective study (25). However, two small RCTs did not show any significant difference comparing restrictive vs. liberal postoperative fluid therapy guided by intrathoracic blood volume index (27,28). One concern is that restricted fluid management protocols could lead to harmful effects such as acute kidney injury. A retrospective study in a mixed cohort of 1,442 patients undergoing either esophageal or pulmonary resection showed that fluid restriction did not lead to postoperative acute kidney injury (21). In comparison, another study showed that patients with a stroke volume index <35 mL/m2 had decreased renal function on postoperative day 1–3 after surgery and increased risk for acute kidney injury (38).
Goal-directed fluid therapy
One Japanese study compared the results of esophagectomy before and after the introduction of a goal-directed fluid therapy protocol using stroke volume variation and stroke volume index to guide fluid therapy. Findings demonstrate earlier gastrointestinal functional recovery and increased serum albumin six months after discharge. There were no significant differences concerning length of stay or complications (39). A similar study from the Netherlands, investigated the results before and after the introduction of a goal-directed fluid management system, based on variation in stroke volume. The patients in the goal-directed therapy group received significantly less fluids but more colloids. There was no difference in overall morbidity or mortality, but there was a decrease in pneumonia, intrathoracic abscesses formation, conduit necrosis, and length of stay in intensive care (40).
Assessment of conduit perfusion
A significant component of the esophagectomy literature has evolved assessing various approaches to improve perioperative conduit health. Indocyanine green angiography and laser Doppler flowmetry are techniques that have been utilized to visualize the circulation in the gastric conduit during surgery (46-49). Preoperative ischemic conditioning of the conduit can be performed by laparoscopic preparation of the conduit 3–7 days before transthoracic esophagectomy, with the aim to improve microcirculation in the proximal conduit and decrease anastomotic leaks (50,51). Theoretically, these techniques can be used to assess and improve the conduit blood flow, optimize the location for the anastomosis, and enhance conduit healing, however none of these techniques have been conclusively demonstrated to improve outcomes in prospective studies, and none have seen widespread clinical application.
Al-Rawi et al. reported a trial in humans that involved suturing Doppler flow probes at the pylorus and the perianastomotic area of the gastric conduit during surgery. Conduit perfusion variation was assessed following an epidural bolus of 0.1 mL/kg of bupivacaine 0.25%. Epidural bolus was shown to significantly decrease conduit perfusion at the proximal (anastomotic) end of the conduit but not in the region of the pylorus. Perfusion to the anastomotic end of the conduit was restored with adrenaline infusion without the need for additional fluid (22). Klijn et al. assessed blood flow in the gastric conduit in a pig model. The application of vasopressors under normovolemic conditions had no detrimental effect on conduit microvascular circulation. The study demonstrates that MAP >70 mmHg was optimal for conduit perfusion but maintaining MAP above 90 mmHg did not lead to any additional improvement in conduit perfusion (30).
Complex invasive and non-invasive methodologies have therefore not been shown to have any substantial beneficial effect on short-term outcomes following esophagectomy. However, there is data to support maintaining a MAP >70 mmHg, and evidence that, when other hemodynamic parameters are stable, vasopressors can be utilized to increase MAP and optimize microvascular perfusion of the anastomosis and conduit.
SHP at the Virginia Mason Medical Center
With the intention to minimize individual variation in patient care during the immediate postoperative period a standardized clinical pathway (SCP) for esophagectomy has been used at the Virginia Mason Medical Center since 1991.Our experience of using this SCP has been presented in two previous publications (34,36). The SCP has evolved over time and the SHP was added in 2004, with its evolution principally influenced by the anesthetic literature.
The SHP at Virginia Mason Medical Center has also evolved over time but has been in its present form since 2010. The SHP provides a framework to set hemodynamic goals, and standardize the immediate post-operative hemodynamic management of patients after esophagectomy. The protocol was embedded within pre-existing SCP and ERAS program and also within the intensive care unit checklists and ordersets. It was based initially, and evolved according, to the best information available at the time documenting the importance of maintaining certain systemic blood pressure levels and then providing a structured framework for response. During the same period that the SHP was introduced patients were subject to fluid restrictive protocols, patient controlled epidural anesthesia (PCEA) and were undergoing attempted mobilization on the day of surgery. One of the most important changes was the elimination of epidural boluses for postoperative pain control, which reduces the risk for immediate postoperative hypotension. Changes in epidural infusion are done through variation of infusion rate and not administration of boluses of narcotic analgesia and local anesthetic.
The SHP established a “best practice” approach to perioperative hypotension and standardized treatment that minimized the potential for variation associated with changing personnel including staff surgeons, nurses and trainees. The protocol was developed with the active participation of the entire multidisciplinary team including anesthesiology, interventionists and intensive care unit nursing and physical therapy. Designated surgical and nursing staff, in the intensive care unit, and the surgical ward monitors the patients for the first 24–48 postoperative hours. Thoracic epidural catheter is the primary choice for initial pain management, using an infusion of bupivacaine 0.05%, and hydromorphone 10-microgram/cc at 8 mL/h. If there is any uncertainty regarding the position of the epidural catheter then an epidurogram is performed before leaving the operating room. The catheter is repositioned or replaced when indicated, to ensure a functioning pain treatment system. Blood transfusions are not given unless a hematocrit <25% is demonstrated in two consecutive measurements or where the patient has a documented history of coronary artery disease. A staff surgeon typically reviews the patient prior to transfusion. The fluid management is conservative, and perioperative fluid administration is aimed toward avoiding weight gain of >2 kg (7). The volume of intraoperative fluid administered in the 501 esophagectomy patients between 2004 and 2018 was on average 2,895 mL with an average length of the procedure being 6.5 hours. Postoperative fluid is set at 0.5 mL/kg/h to a maximum of 100 mL/h, MAP is maintained >70 mmHg with the use of phenylephrine when indicated. Colloids are not used unless specific indications exist. The order sets require a clinical review when a patient has a MAP <70 mmHg for more than 15 minutes. The “in house” managing clinical team has the SHP available for review within the postoperative order sets (Table 2). Since the introduction of the SHP in 2004, the complication rate is 53.2%, anastomotic leak rate 5.2%, and conduit necrosis rate 0%. These outcomes compare favorably with outcomes published by the ECCG where complication rates were 59%, anastomotic leak rate 11.4% and conduit necrosis 1.3% (9).

Full table
Discussion
SCPs have been demonstrated to improve outcomes, of which SHPs are an important component (34,36,52). The current surgical literature has considered a number of methods for invasive and non-invasive perioperative monitoring and has assessed various approaches to fluid utilization both intra- and postoperatively (53-58). No consensus can be reached that any of these interventions significantly improve outcomes although restrictive or goal-directed fluid protocols are currently recommended per ERAS guidelines (7). Fluid restriction with the aim to maintain the patients’ preoperative weight in the immediate postoperative period, is included in many enhanced recovery pathways, since it has been shown to decrease the risk for total postoperative complications, cardiopulmonary complications, and tissue healing in colorectal cancer surgery and other abdominal surgeries (7,8,59-67).
Perioperative fluids are administered to treat hypotension during surgery caused by anesthesia and epidural induced vascular dilatation, bleeding and insensible losses. The use of vasopressors has been identified as a potential risk for decreased conduit circulation (68), but studies have shown that intravenous adrenaline, phenylephrine, or ephedrine can increase MAP and maintain conduit perfusion as long as the patient is otherwise hemodynamically stable (22,69-71). Fluid overload can threaten anastomotic integrity and confer increased risk for postoperative morbidity and mortality (72-74). Goal-directed therapy is a fluid treatment approach that focuses on physiological output measurements (75-78). Stroke volume variation monitoring has been shown to reduce the number of hypotensive events and lactate levels during major abdominal surgery, and to decrease the risk for postoperative complications (79). A meta-analysis including 4188 patients with major surgery showed significantly decreased risk for postoperative infections and pneumonia with the use of goal-directed therapy or routine hemodynamic protocol practice (52).
Anastomotic leak and conduit necrosis remain the most problematic issues following esophagectomy. Leak rates even in high volume centers remain over 11% and conduit necrosis between 1–2% (9). Both of these complications affect perioperative mortality, length of stay, HRQOL and costs. Although very little (7) has been published on SHP, we have utilized an SHP in our SCP since 2004. During that period the anastomotic leak rate has been 5% and we have not experienced any incidence of clinically significant necrosis in over 500 consecutive resections. An SHP does not remove the requirements for individual assessment and clinical judgment. It does provide specific targeted goals for perioperative monitoring and a structured framework for response. This is increasingly important in situations where staff turnover is a regular feature even in high volume centers. These SHPs need to be imbedded within ordersets and the evolution needs to involve all major stakeholders, including nursing, anesthesia, intensivists and trainees. Applying a SHP will make the conduit less prone to misadventure.
Acknowledgements
Ryan Hill Research Foundation supported this work.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Mariette C, Piessen G, Triboulet JP. Therapeutic strategies in oesophageal carcinoma: role of surgery and other modalities. Lancet Oncol 2007;8:545-53. [Crossref] [PubMed]
- Derogar M, Lagergren P. Health-related quality of life among 5-year survivors of esophageal cancer surgery: a prospective population-based study. J Clin Oncol 2012;30:413-8. [Crossref] [PubMed]
- Derogar M, Orsini N, Sadr-Azodi O, et al. Influence of major postoperative complications on health-related quality of life among long-term survivors of esophageal cancer surgery. J Clin Oncol 2012;30:1615-9. [Crossref] [PubMed]
- Paul S, Altorki N. Outcomes in the management of esophageal cancer. J Surg Oncol 2014;110:599-610. [Crossref] [PubMed]
- Markar SR, Karthikesalingam A, Low DE. Enhanced recovery pathways lead to an improvement in postoperative outcomes following esophagectomy: systematic review and pooled analysis. Dis Esophagus 2015;28:468-75. [Crossref] [PubMed]
- Feldheiser A, Aziz O, Baldini G, et al. Enhanced Recovery After Surgery (ERAS) for gastrointestinal surgery, part 2: consensus statement for anaesthesia practice. Acta Anaesthesiol Scand 2016;60:289-334. [Crossref] [PubMed]
- Low DE, Allum W, De Manzoni G, et al. Guidelines for Perioperative Care in Esophagectomy: Enhanced Recovery After Surgery (ERAS®) Society Recommendations. World J Surg 2019;43:299-330. [Crossref] [PubMed]
- Findlay JM, Gillies RS, Millo J, et al. Enhanced recovery for esophagectomy: a systematic review and evidence-based guidelines. Ann Surg 2014;259:413-31. [Crossref] [PubMed]
- Low DE, Kuppusamy MK, Alderson D, et al. Benchmarking Complications Associated with Esophagectomy. Ann Surg 2019;269:291-8. [Crossref] [PubMed]
- Markar S, Gronnier C, Duhamel A, et al. Pattern of Postoperative Mortality After Esophageal Cancer Resection According to Center Volume: Results from a Large European Multicenter Study. Ann Surg Oncol 2015;22:2615-23. [Crossref] [PubMed]
- Briel JW, Tamhankar AP, Hagen JA, et al. Prevalence and risk factors for ischemia, leak, and stricture of esophageal anastomosis: gastric pull-up versus colon interposition. J Am Coll Surg 2004;198:536-41; discussion 541-2. [Crossref] [PubMed]
- Larburu Etxaniz S, Gonzales Reyna J, Elorza Orue JL, et al. Cervical anastomotic leak after esophagectomy: diagnosis and management. Cir Esp 2013;91:31-7. [Crossref] [PubMed]
- Markar S, Gronnier C, Duhamel A, et al. The Impact of Severe Anastomotic Leak on Long-term Survival and Cancer Recurrence After Surgical Resection for Esophageal Malignancy. Ann Surg 2015;262:972-80. [Crossref] [PubMed]
- Sauvanet A, Mariette C, Thomas P, et al. Mortality and morbidity after resection for adenocarcinoma of the gastroesophageal junction: predictive factors. J Am Coll Surg 2005;201:253-62. [Crossref] [PubMed]
- Turkyilmaz A, Eroglu A, Aydin Y, et al. The management of esophagogastric anastomotic leak after esophagectomy for esophageal carcinoma. Dis Esophagus 2009;22:119-26. [Crossref] [PubMed]
- Klevebro F, Friesland S, Hedman M, et al. Neoadjuvant chemoradiotherapy may increase the risk of severe anastomotic complications after esophagectomy with cervical anastomosis. Langenbecks Arch Surg 2016;401:323-31. [Crossref] [PubMed]
- Juloori A, Tucker SL, Komaki R, et al. Influence of preoperative radiation field on postoperative leak rates in esophageal cancer patients after trimodality therapy. J Thorac Oncol 2014;9:534-40. [Crossref] [PubMed]
- Vande Walle C, Ceelen WP, Boterberg T, et al. Anastomotic complications after Ivor Lewis esophagectomy in patients treated with neoadjuvant chemoradiation are related to radiation dose to the gastric fundus. Int J Radiat Oncol Biol Phys 2012;82:e513-9. [Crossref] [PubMed]
- Choudhuri AH, Uppal R, Kumar M. Influence of non-surgical risk factors on anastomotic leakage after major gastrointestinal surgery: Audit from a tertiary care teaching institute. Int J Crit Illn Inj Sci 2013;3:246-9. [Crossref] [PubMed]
- Fumagalli U, Melis A, Balazova J, et al. Intra-operative hypotensive episodes may be associated with post-operative esophageal anastomotic leak. Updates Surg 2016;68:185-90. [Crossref] [PubMed]
- Ahn HJ, Kim JA, Lee AR, et al. The Risk of Acute Kidney Injury from Fluid Restriction and Hydroxyethyl Starch in Thoracic Surgery. Anesth Analg 2016;122:186-93. [Crossref] [PubMed]
- Al-Rawi OY, Pennefather SH, Page RD, et al. The effect of thoracic epidural bupivacaine and an intravenous adrenaline infusion on gastric tube blood flow during esophagectomy. Anesth Analg 2008;106:884-7. table of contents. [Crossref] [PubMed]
- Buise M, Van Bommel J, Mehra M, et al. Pulmonary morbidity following esophagectomy is decreased after introduction of a multimodal anesthetic regimen. Acta Anaesthesiol Belg 2008;59:257-61. [PubMed]
- Casado D, Lopez F, Marti R. Perioperative fluid management and major respiratory complications in patients undergoing esophagectomy. Dis Esophagus 2010;23:523-8. [Crossref] [PubMed]
- Glatz T, Kulemann B, Marjanovic G, et al. Postoperative fluid overload is a risk factor for adverse surgical outcome in patients undergoing esophagectomy for esophageal cancer: a retrospective study in 335 patients. BMC Surg 2017;17:6. [Crossref] [PubMed]
- Haas S, Eichhorn V, Hasbach T, et al. Goal-directed fluid therapy using stroke volume variation does not result in pulmonary fluid overload in thoracic surgery requiring one-lung ventilation. Crit Care Res Pract 2012;2012:687018. [Crossref] [PubMed]
- Haase O, Raue W, Neuss H, et al. Influence of postoperative fluid management on pulmonary function after esophagectomy. Acta Chir Belg 2013;113:415-22. [Crossref] [PubMed]
- Karaman Ilic M, Madzarac G, Kogler J, et al. Intraoperative volume restriction in esophageal cancer surgery: an exploratory randomized clinical trial. Croat Med J 2015;56:290-6. [Crossref] [PubMed]
- Kita T, Mammoto T, Kishi Y. Fluid management and postoperative respiratory disturbances in patients with transthoracic esophagectomy for carcinoma. J Clin Anesth 2002;14:252-6. [Crossref] [PubMed]
- Klijn E, Niehof S, de Jonge J, et al. The effect of perfusion pressure on gastric tissue blood flow in an experimental gastric tube model. Anesth Analg 2010;110:541-6. [Crossref] [PubMed]
- Kobayashi M, Ko M, Kimura T, et al. Perioperative monitoring of fluid responsiveness after esophageal surgery using stroke volume variation. Expert Rev Med Devices 2008;5:311-6. [Crossref] [PubMed]
- Kobayashi M, Koh M, Irinoda T, et al. Stroke volume variation as a predictor of intravascular volume depression and possible hypotension during the early postoperative period after esophagectomy. Ann Surg Oncol 2009;16:1371-7. [Crossref] [PubMed]
- Kuppusamy MK, Felisky CD, Helman JD, et al. Assessment of intra-operative haemodynamic changes associated with transhiatal and transthoracic oesophagectomy. Eur J Cardiothorac Surg 2010;38:665-8. [Crossref] [PubMed]
- Low DE, Kunz S, Schembre D, et al. Esophagectomy--it's not just about mortality anymore: standardized perioperative clinical pathways improve outcomes in patients with esophageal cancer. J Gastrointest Surg 2007;11:1395-402; discussion 1402. [Crossref] [PubMed]
- Motoyama S, Kitamura M, Kibira S, et al. Does central venous pressure reflect the circulating blood volume for the decrement of compliance just after esophagectomy? Surg Today 2000;30:11-5. [Crossref] [PubMed]
- Neal JM, Wilcox RT, Allen HW, et al. Near-total esophagectomy: the influence of standardized multimodal management and intraoperative fluid restriction. Reg Anesth Pain Med 2003;28:328-34. [PubMed]
- Nishi M, Hiramatsu Y, Hioki K, et al. Risk factors in relation to postoperative complications in patients undergoing esophagectomy or gastrectomy for cancer. Ann Surg 1988;207:148-54. [Crossref] [PubMed]
- Sugasawa Y, Hayashida M, Yamaguchi K, et al. Usefulness of stroke volume index obtained with the FloTrac/ Vigileo system for the prediction of acute kidney injury after radical esophagectomy. Ann Surg Oncol 2013;20:3992-8. [Crossref] [PubMed]
- Taniguchi H, Sasaki T, Fujita H, et al. Effects of goal-directed fluid therapy on enhanced postoperative recovery: An interventional comparative observational study with a historical control group on oesophagectomy combined with ERAS program. Clin Nutr ESPEN 2018;23:184-93. [Crossref] [PubMed]
- Veelo DP, van Berge Henegouwen MI, Ouwehand KS, et al. Effect of goal-directed therapy on outcome after esophageal surgery: A quality improvement study. PLoS One 2017;12:e0172806. [Crossref] [PubMed]
- Wei S, Tian J, Song X, et al. Association of perioperative fluid balance and adverse surgical outcomes in esophageal cancer and esophagogastric junction cancer. Ann Thorac Surg 2008;86:266-72. [Crossref] [PubMed]
- Xu WY, Wang N, Xu HT, et al. Effects of sevoflurane and propofol on right ventricular function and pulmonary circulation in patients undergone esophagectomy. Int J Clin Exp Pathol 2013;7:272-9. [PubMed]
- Zou YB, Yan H, Liu XH, et al. Lateral position could provide more excellent hemodynamic parameters during video-assisted thoracoscopic esophagectomy for cancer. Surg Endosc 2013;27:3720-5. [Crossref] [PubMed]
- Watson X, Cecconi M. Haemodynamic monitoring in the peri-operative period: the past, the present and the future. Anaesthesia 2017;72 Suppl 1:7-15. [Crossref] [PubMed]
- Cannesson M. Arterial pressure variation and goal-directed fluid therapy. J Cardiothorac Vasc Anesth 2010;24:487-97. [Crossref] [PubMed]
- Karampinis I, Ronellenfitsch U, Mertens C, et al. Indocyanine green tissue angiography affects anastomotic leakage after esophagectomy. A retrospective, case-control study. Int J Surg 2017;48:210-4. [Crossref] [PubMed]
- Campbell C, Reames MK, Robinson M, et al. Conduit Vascular Evaluation is Associated with Reduction in Anastomotic Leak After Esophagectomy. J Gastrointest Surg 2015;19:806-12. [Crossref] [PubMed]
- Schilling MK, Redaelli C, Maurer C, et al. Gastric microcirculatory changes during gastric tube formation: assessment with laser Doppler flowmetry. J Surg Res 1996;62:125-9. [Crossref] [PubMed]
- Ambrus R, Svendsen LB, Secher NH, et al. A reduced gastric corpus microvascular blood flow during Ivor-Lewis esophagectomy detected by laser speckle contrast imaging technique. Scand J Gastroenterol 2017;52:455-61. [Crossref] [PubMed]
- Holscher AH, Schneider PM, Gutschow C, et al. Laparoscopic ischemic conditioning of the stomach for esophageal replacement. Ann Surg 2007;245:241-6. [Crossref] [PubMed]
- Nguyen NT, Longoria M, Sabio A, et al. Preoperative laparoscopic ligation of the left gastric vessels in preparation for esophagectomy. Ann Thorac Surg 2006;81:2318-20. [Crossref] [PubMed]
- Dalfino L, Giglio MT, Puntillo F, et al. Haemodynamic goal-directed therapy and postoperative infections: earlier is better. A systematic review and meta-analysis. Crit Care 2011;15:R154. [Crossref] [PubMed]
- Chong PC, Greco EF, Stothart D, et al. Substantial variation of both opinions and practice regarding perioperative fluid resuscitation. Can J Surg 2009;52:207-14. [PubMed]
- Carney A, Dickinson M. Anesthesia for esophagectomy. Anesthesiol Clin 2015;33:143-63. [Crossref] [PubMed]
- Chau EH, Slinger P. Perioperative fluid management for pulmonary resection surgery and esophagectomy. Semin Cardiothorac Vasc Anesth 2014;18:36-44. [Crossref] [PubMed]
- Joshi GP. Intraoperative fluid restriction improves outcome after major elective gastrointestinal surgery. Anesth Analg 2005;101:601-5. [Crossref] [PubMed]
- Umari M, Falini S, Segat M, et al. Anesthesia and fast-track in video-assisted thoracic surgery (VATS): from evidence to practice. J Thorac Dis 2018;10:S542-54. [Crossref] [PubMed]
- Veelo DP, Geerts BF. Anaesthesia during oesophagectomy. J Thorac Dis 2017;9:S705-12. [Crossref] [PubMed]
- Brandstrup B, Tonnesen H, Beier-Holgersen R, et al. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg 2003;238:641-8. [Crossref] [PubMed]
- Lobo DN, Bostock KA, Neal KR, et al. Effect of salt and water balance on recovery of gastrointestinal function after elective colonic resection: a randomised controlled trial. Lancet 2002;359:1812-8. [Crossref] [PubMed]
- Nisanevich V, Felsenstein I, Almogy G, et al. Effect of intraoperative fluid management on outcome after intraabdominal surgery. Anesthesiology 2005;103:25-32. [Crossref] [PubMed]
- Lobo SM, Ronchi LS, Oliveira NE, et al. Restrictive strategy of intraoperative fluid maintenance during optimization of oxygen delivery decreases major complications after high-risk surgery. Crit Care 2011;15:R226. [Crossref] [PubMed]
- Mayer J, Boldt J, Mengistu AM, et al. Goal-directed intraoperative therapy based on autocalibrated arterial pressure waveform analysis reduces hospital stay in high-risk surgical patients: a randomized, controlled trial. Crit Care 2010;14:R18. [Crossref] [PubMed]
- Bundgaard-Nielsen M, Holte K, Secher NH, et al. Monitoring of peri-operative fluid administration by individualized goal-directed therapy. Acta Anaesthesiol Scand 2007;51:331-40. [Crossref] [PubMed]
- Pearse R, Dawson D, Fawcett J, et al. Early goal-directed therapy after major surgery reduces complications and duration of hospital stay. A randomised, controlled trial Crit Care 2005;9:R687-93. [ISRCTN38797445]. [Crossref] [PubMed]
- Pearse RM, Harrison DA, MacDonald N, et al. Effect of a perioperative, cardiac output-guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: a randomized clinical trial and systematic review. JAMA 2014;311:2181-90. [Crossref] [PubMed]
- Voldby AW, Brandstrup B. Fluid therapy in the perioperative setting-a clinical review. J Intensive Care 2016;4:27. [Crossref] [PubMed]
- Theodorou D, Drimousis PG, Larentzakis A, et al. The effects of vasopressors on perfusion of gastric graft after esophagectomy. An experimental study. J Gastrointest Surg 2008;12:1497-501. [Crossref] [PubMed]
- Jansen SM, de Bruin DM, van Berge Henegouwen MI, et al. Effect of ephedrine on gastric conduit perfusion measured by laser speckle contrast imaging after esophagectomy: a prospective in vivo cohort study. Dis Esophagus 2018.31. [PubMed]
- Pathak D, Pennefather SH, Russell GN, et al. Phenylephrine infusion improves blood flow to the stomach during oesophagectomy in the presence of a thoracic epidural analgesia. Eur J Cardiothorac Surg 2013;44:130-3. [Crossref] [PubMed]
- Holte K, Foss NB, Svensen C, et al. Epidural anesthesia, hypotension, and changes in intravascular volume. Anesthesiology 2004;100:281-6. [Crossref] [PubMed]
- Malhotra K, Axisa B. Low plasma albumin linked to fluid overload in postoperative epidural patients. Ann R Coll Surg Engl 2009;91:703-7. [Crossref] [PubMed]
- Moller AM, Pedersen T, Svendsen PE, et al. Perioperative risk factors in elective pneumonectomy: the impact of excess fluid balance. Eur J Anaesthesiol 2002;19:57-62. [Crossref] [PubMed]
- Marjanovic G, Villain C, Juettner E, et al. Impact of different crystalloid volume regimes on intestinal anastomotic stability. Ann Surg 2009;249:181-5. [Crossref] [PubMed]
- Bjerregaard LS, Moller-Sorensen H, Hansen KL, et al. Using clinical parameters to guide fluid therapy in high-risk thoracic surgery. A retrospective, observational study. BMC Anesthesiol 2015;15:91. [Crossref] [PubMed]
- Jhanji S, Vivian-Smith A, Lucena-Amaro S, et al. Haemodynamic optimisation improves tissue microvascular flow and oxygenation after major surgery: a randomised controlled trial. Crit Care 2010;14:R151. [Crossref] [PubMed]
- Chappell D, Jacob M, Hofmann-Kiefer K, et al. A rational approach to perioperative fluid management. Anesthesiology 2008;109:723-40. [Crossref] [PubMed]
- Durkin C, Schisler T, Lohser J. Current trends in anesthesia for esophagectomy. Curr Opin Anaesthesiol 2017;30:30-5. [PubMed]
- Benes J, Chytra I, Altmann P, et al. Intraoperative fluid optimization using stroke volume variation in high risk surgical patients: results of prospective randomized study. Crit Care 2010;14:R118. [Crossref] [PubMed]