Results of concomitant groin-free percutaneous temporary RVAD support using a centrifugal pump with a double-lumen jugular venous cannula in LVAD patients
Introduction
The prevalence of heart failure (HF) is continuously increasing and treatment of HF has become a main focus in clinical medicine. Though optimized medical treatment may improve survival for conservative therapy, heart transplantation (HTx) remains the gold standard in the treatment of end stage HF. Nevertheless, donor organ shortage and/or contraindications for HTx exclude a relevant number of patients from this life-saving therapy. Left ventricular assist devices (LVAD) allows for immediate treatment of severe HF, with two main indications: bridge-to-transplantation (BTT) or destination therapy (DT). Implantation numbers are steadily rising with promising mid- and long-term results (1,2).
Nevertheless, LVAD therapy possesses certain limitations. These can be divided into early (within 30 days postoperatively) and late postoperative adverse clinical events. Pump thrombosis, severe bleeding, development of arterio-venous malformations or aortic regurgitation and driveline-infections represent major late adverse clinical events. In contrast, the most critical early postoperative complication is right HF (RHF), causing a cascade of multi-organ failure (MOF). Hence, RHF is known to nearly double the probability for death within 1 year of LVAD support, mainly due an increase in early post-operative mortality (3).
Addressing the impact of postoperative RHF following LVAD implantation, serval attempts have been made to provide better understanding and to predict the risk of RHF development (3-6). Nevertheless, Kalogeropoulos et al. evaluated the existing predictive tests through external validation, showing merely a modest performance across currently available scores, with an average discrimination value of about 0.60. Despite existing scores and growing experience, RHF still occurs in up to 50% of all LVAD cases (7-9).
Though LVAD therapy usually increases cardiac output immediately, the maladaptation of the right ventricular (RV) preload and afterload may limit the early hemodynamical improvements after implantation. While continuous decrease of RV afterload due to the sufficient left ventricular unloading improves RV function, the increase in RV preload, as it occurs straight after LVAD initiation, can overstrain the RV. Moreover, surgical trauma and volume shift during surgery can additionally increase RV workload (10). Peripheral veno-arterial support can be established relatively simple. Riebandt et al. recently published their experience in the application of a peripheral veno-arterial extracorporeal membrane oxygenation (ECMO) treatment for RHF with acceptable outcome (11). However, veno-arterial support may be overtreatment for RHF and LVAD in place and might cause severe complications, including vessel injuries or peripheral ischemia.
Supporting the RV is especially impeded by accessibility to the pulmonary artery (PA). Different techniques for temporary RV support (t-RVAD) are described in literature. Most techniques follow draining the right atrium (RA) through the femoral vein and returning into the PA. Schopka et al. described their technique using a graft to the PA channelled outside the thorax, avoiding re-thoracotomy in case of successful t-RVAD weaning, by ligating the graft and retaining it subcutaneously (12). Others modified this strategy, adapting it to the minimally invasive LVAD implantation technique (13). Nevertheless, these techniques need either a re-thoracotomy in the operation room or are associated with a further retention of prosthetic material in the chest. In addition, these strategies of t-RVAD come with a severe limitation of difficult mobilization of patients on support, with consecutively associated complications. These complications my also contribute to another certain limitation in the results of temporary RVAD, as successful weaning is considerably limited in those patients (14,15).
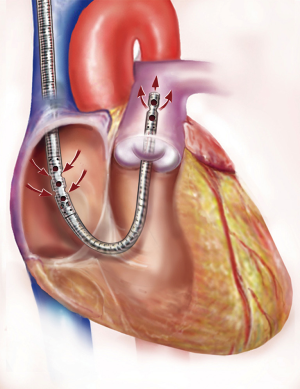
We previously described our technique of t-RVAD using the jugular approach of the ProtekDuo™ dual-lumen cannula in combination with the TandemHeart™ centrifugal pump via the right jugular vein in analogy to a Swan-Ganz catheter (10). The groin-free, transjugular placement of the ProtekDuo™ dual-lumen canula is presented schematically in Figure 1. The advantage of this technique is providing full RV support through an entirely percutaneous approach. Therefore, our groin-free approach allows for full mobilization and a complete bed-side explantation strategy without the need for re-operation, let alone re-sternotomy. In case of lung failure, an upgrade with an oxygenator is also feasible and safe.
We herein report the results of our novel technique providing various advantages as compared to traditional RA-PA approaches.
Methods
Patients
This is retrospective, single centre observational study. Between October 2015 and September 2017, a total of 11 patients with symptomatic end-stage HF underwent permanent LVAD implantation (HeartWare HVAD™, Medtronic HeartWare, Inc., Framingham, MA, USA or Abbott St. Jude Medical Heartmate III™, St. Jude Medical, Saint Paul, MN, USA) and concomitant t-RVAD implantation using the TandemLife TandemHeart™ plus ProtekDuo™ dual-lumen cannula (LivaNova, PLC, London, UK). Patient selection and indication for LVAD implantation followed the present international recommendation for permanent mechanical circulatory support (MCS) (16). Every case underwent obligatory interdisciplinary approval by the institutional heart team, consisting of a HF cardiologist, cardio-thoracic surgeon, anaesthesiologist and a specialist in psychosomatic medicine.
This work complies with the declaration of Helsinki. The study was approved by the ethical committee of the Medical School, University Heidelberg (S-708/2017).
Implantation procedure
LVAD implantation was performed in a standardized fashion with a full sternotomy approach using cardiopulmonary bypass (CPB) in all cases. After successful LVAD placement and completion of the LVAD outflow graft anastomosis to the ascending aorta, the t-RVAD implantation using the TandemHeart™ with the ProtekDuo™ cannula followed. As we have described previously, under fluoroscopic and TEE control, a Swan-Ganz catheter was placed over a pre-bend Super Stiff guide-wire into the PA via the right jugular vein using Seldinger technique. After removal of the Swan-Ganz catheter, the dual-lumen ProtekDuo™ cannula was inserted into the main PA over the guide wire followed by the removal of the guide wire (10). Upon initiation of LVAD support, RVAD support was initiated and weaning from CPB was performed by gradually decreasing CPB flow and increasing t-RVAD flow to a maximum of 7,500 rpm (up to 3.6 L/min). Complete post-procedure heparin reversal was performed in all cases to secure haemostasis. Target activated partial thromboplastin time (aPTT) was 60–80 s. with a point-of-care activated clotting time of 180–220 s. Per design, the TandemHeart™ pump mandatorily needs to be flushed with saline. No additional post-operative anticoagulation was needed.
t-RVAD weaning protocol
t-RVAD support was evaluated daily according to organ function, need for inotropic support and/or inhaled nitric oxide (iNO) and patients’ clinical status by cardio-thoracic intensive care specialists on a specialized cardio-thoracic intensive care unit (ICU). Extubation was done as early as possible. t-RVAD support was gradually decreased by 500–1,000 rpm/day (corresponding to about 0.25–0.50 litres/minute) until the minimal TandemHeart™ rpm of 3,500 rpm was reached. On 3,500 rpm, bedside evaluation of the patient including transthoracic echocardiography (TTE) was performed to validate safety of t-RVAD termination. Evaluation of the right heart function was performed by daily echocardiography and monitoring of central venous saturation as well as end-organ function. During the weaning process, all patients were on low-dose dobutamine or milrinone/levosimendan depending on peripheral vascular resistance.
t-RVAD explantation was performed bedside on ICU under local anaesthesia by simply stopping the TandemHeart™ and extracting the ProtekDuo™ cannula out of the jugular vein in analogy to the removal of a central venous line. Apart from the placement of a temporary skin suture, no further surgical intervention became necessary.
Statistics
Statistical analysis was processed using the Statistical Package for Social Sciences version 24 (SPSS, Inc., Chicago, IL, USA). Variables are given as continuous or categorical variables. Continuous data were shown as mean ± standard deviation. Wilcoxon signed-rank test was utilized for categorical variables. Kaplan-Meier analysis was used to estimate survival function.
Results
Patient characteristics and preoperative condition
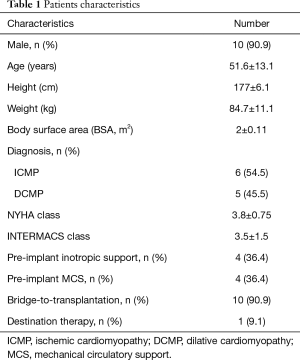
Between October 2015 and September 2017, 11 patients, who underwent LVAD implantation for severe HF, received a t-RVAD (91% male, mean age 51.6±13.1 years) due to impaired RV function. Preoperative functional NYHA class was 3.8±0.75 and patients were classified as INTERMACS 3.5±1.5. Underlying diseases for end-stage HF were ischemic (54.5%) and dilative cardiomyopathies (45.5%), respectively. Of all 11 patients, 4 (36.4%) were in need for inotropic support before surgical treatment and MCS was already established in 4 (36.4%) patients with 2 (18.2%) patients on preoperative peripheral extracorporeal membrane oxygenation (ECMO) and 1 (9.1%) patient on temporary LVAD support (TandemHeart™ percutaneous LVAD) before the permanent LVAD implantation procedure. Intention to treatment was BTT in 10 patients (90.9%) and DT in one patient (9.1%). Baseline demographics are detailed in Table 1.
Full table
Preoperative echocardiography evaluation, RV assessment and laboratory findings
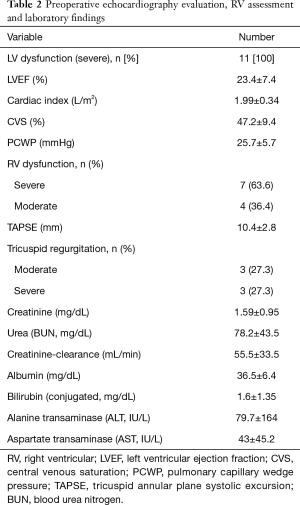
Preoperative mean ejection fraction (EF) of the LV was 23.4%±7.4%. Seven patients (63.6%) suffered from severely reduced, and 4 patients (36.4%) from moderately reduced RV function. Mean tricuspid annular plane systolic excursion (TAPSE) was 10.4±2.8 mm. Echocardiography showed moderate tricuspid regurgitation (TR) in 3 patients (27.3%) and severe TR in another 3 patients (27.3%). Preoperative cardiac index (CI) was calculated at 1.99±0.34 L/m2 during right heart catheterization. Central venous saturation was 47.2%±9.4% and pulmonary capillary wedge pressure was measured at 25.7±5.7 mmHg. Laboratory findings showed increased kidney and liver function parameters already indicating impaired end-organ function. Table 2 shows preoperative echocardiography, right heart catheterization values and laboratory findings.
Full table
Procedural characteristics
Nine patients (81.8%) underwent urgent surgery. Mean operation time was 311±26 min. All procedures were performed on CPB support. Mean CPB time was 159.3±22 min. HeartWare (HVAD™) were used in 6 patients (54.5%), while 5 (45.5%) received an Abbott HeartMate III™ permanent LVAD. The standard access was achieved via median sternotomy (100%). Three (27.3%) surgical procedures were performed as re-do surgeries. Concomitant procedures, were done in 2 patients (18.2%), who received additional closure of atrial septal defects. In one patient (9.1%), the addition of a membranous oxygenator to the t-RVAD circuit was needed. Details are presented in Table 3.
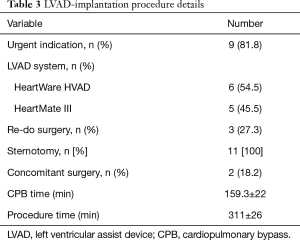
Full table
Clinical outcome data
Mean ICU stay were 23.8±16.5 days. Per institutional protocol postoperative nitric oxide (NO) ventilation was routinely installed in all patients (100%) with a mean NO ventilation time of 117±123 h. Overall mechanical ventilation time was 265±381 h. Mean support duration was 16.8±9.5 days. Ten patients (90.9%) could be successfully weaned from t-RVAD, one patient died from MOF on on-going t-RVAD 17 days after surgery (POD).
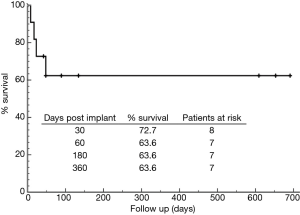
Thirty-day survival was 72.7%. Follow-up was 100% complete with 214.7±283 days. In total 4 patients died during follow-up, 3 (27.3%) due to cardiovascular related MOF (POD 8, 17 & 48) and 1 patient (9.1%) as a result of a fatal intracranial bleed (ICB) 12 days after t-RVAD explantation. T-RVAD and outcome results are presented in Table 4, for the Kaplan-Meier survival curve, please see Figure 2. There were no RVAD associated complications and all patients were mobilized on t-RVAD support.
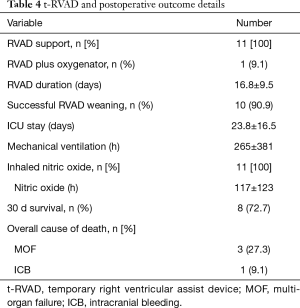
Full table
Discussion
In this paper we describe the first results of one-staged groin-free RV-support in patients receiving an LVAD. Postoperative RVF is a key factor in the determination of a successful or failed LVAD therapy. Recent successes in the field of LVAD therapy and growing experience with the technique have pushed the boundaries towards sicker recipients and patients presenting with an impaired RVF at time of LVAD implantation. However, RHF following LVAD implantation remains to be a significant limitation resulting in a relevant morbidity and significant mortality (3,5,17).
In the context of t-RVAD, two major issues are continuously discussed controversially: (I) if/when to apply a t-RVAD; and (II) how to apply a t-RVAD.
Existing risk stratification models
Multiple parameters have been described to predict RVF after LVAD including laboratory parameters, echocardiography measurements and clinical variables. However, assessment of RV-function prior to LVAD implantation using existing scores remains limited. The proportion of advantageous RV unloading contribution to an overall LVAD success is barely reliable with only modest performance of all existing scores in the prediction of RVF at the most (7). While the recently published EUROMACS right-sided HF risk score outperforms earlier models, however, all scores remain based on retrospective analysis with pending external validation (18).
To support or not to support—timing of t-RVAD
It remains debatable, when a t-RVAD should be applied best in the circumstance of LVAD implantation and RVF. As previously discussed, RVF may appear even if pre-implantation assessment does not suggest high risk, e.g., following intraoperative complication, bleeding causing a relevant shift of volume, etc. While there is little doubt that a preserved RV capacity allows for LVAD implantation alone, every alteration of the RV might result in concerns whether a t-RVAD will become necessary. In addition, prolonged procedure time and/or the need for concomitant surgery (including the need for aortic cross clamp, cardioplegic arrest and myocardial ischemia) might aggravate a marginal RV dysfunction.
Ravichandran et al. published their initial experience using the TandemHeart/ProtekDuo t-RVAD. Given the heterogenous cohort of this publication, success was limited with a rate of successful weaning in only 23% and 41% of the patients died from RHF (19).
Advantages and disadvantages of previously described techniques for t-RVAD
It remains a subject of debate, how a t-RVAD may be established best to provide sufficient pulmonary blood flow and to prevent a right-to-left forward failure. Different techniques have been described before to establish a temporary right heart bypass to partially exclude the RV. While venous drainage is relatively easy to install by placing a multi-stage cannula into the femoral vein by a groin approach, bypassing the right ventricle is more difficult. The standard approach to reach the PA is direct cannulation in case of open sternotomy. Explantation then requires a re-sternotomy in those cases. However, Schopka et al. described their technique of t-RVAD by draining via the femoral vein and sewing a vascular graft to the PA cannulated with an arterial cannula. This graft was channelled outside the thorax allowing for explantation without a re-thoracotomy by removing the cannula from the graft followed by sewing the graft and pushing it back into the chest, once t-RVAD was obsolete (12). Saeed et al. published their experience with a comparable technique in LVAD patients requiring t-RVAD and in cases of post-cardiotomy RVF (20). In times of minimally-invasive LVAD implantation technique avoiding a full sternotomy, Schaefer et al. published their technique of a minimally-invasive t-RVAD implantation technique by adding a left-sided mini-thoracotomy in the 2nd intercostal space to reach the PA (13). Nevertheless, all techniques described above share two major disadvantages. First, all these approaches need venous drainage by placing a large venous cannula into the groin vessel. Though, drainage is excellent, it precludes the patient from a sufficient mobilisation. Decidedly, it is well understood that early mobilization is a crucial factor in the rehabilitation of patients after surgery (21). Within our cohort, all patients were mobilized successfully due to the groin-free approach. Second, all procedures previously described need a second surgery, however some authors describe the explantation procedure is less invasive and achievable on ICU without the need for a general anaesthesia (12,13).
However, there are more technical advantages of the presented t-RVAD system. In cases of LVAD implantation over a median sternotomy and postoperative hemorrhage at the site of apical LVAD insertion, our approach allows for lifting the heart and bleeding treatment without compromising RV-function and the need for re-initiation of CPB.
For centers preferring LVAD implantation over a mini-sternotomy and anterolateral thoracotomy, our approach offers an elegant solution for the treatment of right HF as the PA is not technically accessible for placement of a RVAD without another thoracotomy site.
Limitations
The present study reflects results of single-centre retrospective study with a limited number of patients involved. However, our promising results are the first published contending the novel approach of a concomitant temporary percutaneous RVAD implantation allowing for full RV support (including lung support if needed) with providing a complete explantation without de novo re-thoracotomy and/or general anaesthesia. Our consecutive cohort was not selected by a certain RVF or RV dysfunction assessment protocol. However, our results will lead to the development of a solid protocol to define patients in need for this RV support strategy within our institution as we believe this reflects an important quality marker.
Conclusions
Undoubtedly, LVAD implantation without any occurrence of RVF or the need of t-RVAD is remains first priority in the setup of permanent MCS. Therefore, reliable and validated prediction tools are in need. In addition, a certain definition of RVF or severe RV-dysfunction should be established in every program to allow for standard operation procedures (SOP). This SOP should start by no later than the beginning of the LVAD implantation to prevent RVF in the very first line. Nevertheless, there is a number of patients with reduced RV function conditioned by left ventricular failure. In the context of the limited results of permanent biventricular support, alternatives must be available in modern HF surgery armamentarium. Our t-RVAD strategy described within this work combines all benefits of a safe, groin-free, transcutaneous full RV support device and allows for full mobilization and atraumatic explantation once t-RVAD is no more required. The approach has proven feasibility and may encourage other groups to accept patients with an impaired RVF for implantation of a permanent LVAD having a sufficient and safe tool for temporary RV support available. Nevertheless, further prospective studies have to follow to advocate this technique in a larger setting.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This work complies with the declaration of Helsinki. The study was approved by the ethical committee of the Medical School, University Heidelberg (S-708/2017).
References
- Kirklin JK, Pagani FD, Kormos RL, et al. Eighth annual INTERMACS report: Special focus on framing the impact of adverse events. J Heart Lung Transplant 2017;36:1080-6. [Crossref] [PubMed]
- Mehra MR, Goldstein DJ, Uriel N, et al. Two-Year Outcomes with a Magnetically Levitated Cardiac Pump in Heart Failure. N Engl J Med 2018;378:1386-95. [Crossref] [PubMed]
- Kormos RL, Teuteberg JJ, Pagani FD, et al. Right ventricular failure in patients with the HeartMate II continuous-flow left ventricular assist device: incidence, risk factors, and effect on outcomes. J Thorac Cardiovasc Surg 2010;139:1316-24. [Crossref] [PubMed]
- Fitzpatrick JR 3rd, Frederick JR, Hsu VM, et al. Risk score derived from pre-operative data analysis predicts the need for biventricular mechanical circulatory support. J Heart Lung Transplant 2008;27:1286-92. [Crossref] [PubMed]
- Matthews JC, Koelling TM, Pagani FD, et al. The right ventricular failure risk score a pre-operative tool for assessing the risk of right ventricular failure in left ventricular assist device candidates. J Am Coll Cardiol 2008;51:2163-72. [Crossref] [PubMed]
- Wang Y, Simon MA, Bonde P, et al. Decision tree for adjuvant right ventricular support in patients receiving a left ventricular assist device. J Heart Lung Transplant 2012;31:140-9. [Crossref] [PubMed]
- Kalogeropoulos AP, Kelkar A, Weinberger JF, et al. Validation of clinical scores for right ventricular failure prediction after implantation of continuous-flow left ventricular assist devices. J Heart Lung Transplant 2015;34:1595-603. [Crossref] [PubMed]
- Lampert BC, Teuteberg JJ. Right ventricular failure after left ventricular assist devices. J Heart Lung Transplant 2015;34:1123-30. [Crossref] [PubMed]
- Lo C, Murphy D, Summerhayes R, et al. Right ventricular failure after implantation of continuous flow left ventricular assist device: analysis of predictors and outcomes. Clin Transplant 2015;29:763-70. [Crossref] [PubMed]
- Schmack B, Weymann A, Popov AF, et al. Concurrent Left Ventricular Assist Device (LVAD) Implantation and Percutaneous Temporary RVAD Support via CardiacAssist Protek-Duo TandemHeart to Preempt Right Heart Failure. Med Sci Monit Basic Res 2016;22:53-7. [Crossref] [PubMed]
- Riebandt J, Haberl T, Wiedemann D, et al. Extracorporeal membrane oxygenation support for right ventricular failure after left ventricular assist device implantation. Eur J Cardiothorac Surg 2018;53:590-5. [Crossref] [PubMed]
- Schopka S, Haneya A, Rupprecht L, et al. Temporary right heart assist: a minimally invasive approach. Artif Organs 2012;36:700-4. [Crossref] [PubMed]
- Schaefer A, Reichart D, Bernhardt AM, et al. Outcomes of Minimally Invasive Temporary Right Ventricular Assist Device Support for Acute Right Ventricular Failure During Minimally Invasive Left Ventricular Assist Device Implantation. ASAIO J 2017;63:546-50. [Crossref] [PubMed]
- Takeda K, Naka Y, Yang JA, et al. Outcome of unplanned right ventricular assist device support for severe right heart failure after implantable left ventricular assist device insertion. J Heart Lung Transplant 2014;33:141-8. [Crossref] [PubMed]
- Yoshioka D, Takayama H, Garan RA, et al. Contemporary outcome of unplanned right ventricular assist device for severe right heart failure after continuous-flow left ventricular assist device insertion. Interact Cardiovasc Thorac Surg 2017;24:828-34. [Crossref] [PubMed]
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129-200. [Crossref] [PubMed]
- Dang NC, Topkara VK, Mercando M, et al. Right heart failure after left ventricular assist device implantation in patients with chronic congestive heart failure. J Heart Lung Transplant 2006;25:1-6. [Crossref] [PubMed]
- Soliman OI, Akin S, Muslem R, et al. Derivation and Validation of a Novel Right-Sided Heart Failure Model After Implantation of Continuous Flow Left Ventricular Assist Devices: The EUROMACS (European Registry for Patients with Mechanical Circulatory Support) Right-Sided Heart Failure Risk Score. Circulation 2018;137:891-906. [Crossref] [PubMed]
- Ravichandran AK, Baran DA, Stelling K, et al. Outcomes with the Tandem Protek Duo Dual-Lumen Percutaneous Right Ventricular Assist Device. ASAIO J 2018;64:570-2. [Crossref] [PubMed]
- Saeed D, Maxhera B, Kamiya H, et al. Alternative right ventricular assist device implantation technique for patients with perioperative right ventricular failure. J Thorac Cardiovasc Surg 2015;149:927-32. [Crossref] [PubMed]
- Schaller SJ, Anstey M, Blobner M, et al. Early, goal-directed mobilisation in the surgical intensive care unit: a randomised controlled trial. Lancet 2016;388:1377-88. [Crossref] [PubMed]