
A novel multimodal approach to treating proximal tracheal obstructions with spray cryotherapy
Introduction
Spray cryotherapy (SCT) ablation of airway obstructions is an evolving field of clinical practice and research in thoracic disease (1). The third generation truFreeze System (CSA Medical, Lexington, MA) is the only SCT device approved for airway use. Using a series of valves to regulate the flow of the liquid nitrogen cryogen from the storage dewar to the exit point at the catheter tip, this current system creates a more even flow of liquid nitrogen without nitrogen gas pocket development. This regulated liquid nitrogen release enables greater stability during the transition to nitrogen gas at the treatment site than prior systems and facilitates uniform elastic recoil of the lung for safe nitrogen gas evacuation.
While results are evident immediately after application with visualization of ice crystal formation and freezing of the area of interest, planning prior to cryogen application poses a unique set of challenges. Liquid nitrogen resides inside the console in a storage dewar. A higher pressure, approximately 19 to 20 PSI, is required to allow the liquid nitrogen to exit the storage dewar. The liquid nitrogen then flows through a series of valves that step down that pressure so that it is delivered through the truFreeze cryospray 7 Fr catheter at low pressure, ~2–4 PSI, before rapidly expanding in gaseous form approximately 700-fold upon exiting the catheter (2). Displacement of oxygen by nitrogen gas and barotrauma from resulting high intrathoracic pressures were possible complications seen with the second generation system (3). Multiple precautions are taken to ensure rapid gas egress, including utilizing an open ventilation system with a standard adult rigid bronchoscope with at least an 8 mm inner diameter, or a large airway, commonly through intubation with an 8.5 mm endotracheal tube (4).
Proximal tracheal lesions present a further technical challenge as using a rigid bronchoscope will obscure the lesion from site, thus preventing targeted application of the cryogen to the area of concern. To address this problem, our institution has successfully employed the use of a laryngeal mask airway (LMA) for visualization of proximal airway lesions in conjunction with placement of an esophageal balloon to prevent gas entry into the gastrointestinal tract. This multimodal approach ensures proper visualization of the targeted treatment area, allows for gas egress through an open ventilation system, and prevents gas entry into the gastrointestinal tract, mitigating against developing the complications reported with SCT. We present two recent cases from our interventional pulmonology clinic demonstrating successful application of our technique for further discussion.
Case presentation
Case 1
A 33-year-old female with a history of a tracheal web located 1.5 cm below her vocal cords was evaluated for recurrent dyspnea, wheezing, and a decrease in her peak flow. She had no other significant medical history, including intubations. Bronchoscopy was recommended for further evaluation and potential treatment. Three months prior, she had undergone argon plasma coagulation therapy to the involved area with initial improvement in her symptoms, but ultimate reoccurrence.
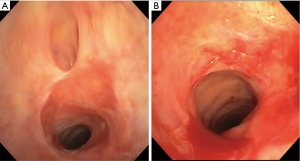
Throughout her procedure, she was maintained under general anesthesia and intubated with a size four LMA. Initial airway inspection revealed a tissue shelf with diverticulum immediately proximal to a tracheal web 1.5 cm below the vocal cords. The tracheal web contained an area of posterior and lateral tissue protrusion which occluded the airway (Figure 1A). A 5.8 mm therapeutic bronchoscope (Olympus, Waltham, MA) was able to be negotiated through the area.
We next placed a 5.5 cm wireguided esophageal balloon dilation catheter (Merit Medical Endotek, South Jordan, UT) into the esophagus with ultrathin bronchoscopy guidance. The balloon was inflated to 12 mm at 8 ATM to fully occlude the esophagus and to prevent any nitrogen gas entry into the gastrointestinal tract. Additionally, a respiratory therapist was asked to hold and to observe the abdomen during SCT activation to monitor for abdominal distention as an additional tactile and visual measure of potential gas egress into the gastrointestinal tract.
With these protective measures in place, the third generation truFreeze cryospray catheter (CSA Medical, Lexington, MA) was used to spray the tracheal web circumferentially twice for a total of five seconds per activation cycle. Before each activation cycle, the LMA was disconnected from the ventilator circuit. Nitrogen gas egress was confirmed by both tactile and visual inspection.
After completing SCT ablation, the airway was visually significantly wider. A CRE wireguided endovention balloon catheter (Boston Scientific, Canton, MA) was then used for sequential two minute dilations to 12, 13.5, and 15 mm with atmospheric pressures of 3, 4.5, and 8, respectively. At procedure completion, a therapeutic bronchoscope was passed with ease through the former tracheal web site (Figure 1B).
Case 2
A 53-year-old male with a recent past medical history of tracheal stenosis due to prolonged intubation was evaluated for persistent dyspnea with exertion and inspiratory wheezing. In the month prior to presentation, he underwent balloon tracheoplasty and CO2 laser treatment three times for treatment, with recurrence of symptoms.
As per our protocol, he was intubated with a size four LMA. Initial airway inspection revealed near circumferential granulation tissue, left greater than right, in the proximal trachea at approximately the second cartilaginous ring (Figure 2A). A 5.8 mm therapeutic bronchoscope was unable to be negotiated pass the stenotic area.
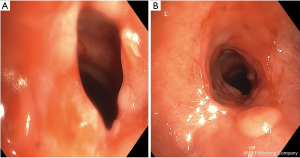
Next, we placed a 5.5 cm wireguided esophageal balloon dilation catheter and employed the same protective measures as detailed above before using the truFreeze cryospray catheter to spray the area of granulation tissue circumferentially four times for a total of 5 seconds per activation cycle. A CRE wireguided endovention balloon catheter was then used for five sequential dilations of 30–45 seconds to 10 mm at 5 ATM. At the end of the procedure, a therapeutic bronchoscope was passed with ease through the stenotic site (Figure 2B).
Discussion
Central airway obstructions from both benign and malignant etiologies are commonly encountered by physicians treating thoracic diseases (5). Fortunately, myriad minimally invasive techniques are now available and commonly performed for treatment and diagnosis (1,6). SCT is a temperature dependent treatment shown to be both safe and feasible for treating central airway obstructions (7). Utilizing flash freezing of tissue at temperatures of −196 °C, SCT results in non-contact cell death with preservation of the extracellular matrix, avoidance of damage to collagen, and regenerative regrowth of tissue (1,6,8).
While prior reports have successfully demonstrated SCT ablation with the use of rigid bronchoscopy for easily accessible airway lesions (2,9,10), proximal airway obstructions pose a significant technical challenge. A recently published case series reported technically successful and sustained response of benign upper and mid tracheal lesions treated with spray cryotherapy, but utilized suspension laryngoscopy to allow for rapid egress after direct application of cryogen therapy (11). Because access to physicians trained in suspension laryngoscopy and to the necessary equipment may be unavailable at some institutions, it is important to develop other techniques to treat these lesions.
Because significant complications, including intraoperative events, pneumothoraces, cardiac arrest, and death, were reported from the initial large volume reports using the second generation SCT system (which was never released for airway use) for central airway obstructions (7,9,10), it is also imperative to employ all possible techniques to mitigate against these events. As these complications are thought to be due to insufficient nitrogen gas egress after its rapid expansion during phase transformation from liquid to gas, it is important to rapidly remove all gas from the airway and prevent its accumulation in other organ systems. While gastroenterology procedures facilitate this rapid gas evacuation through the active use of a suction catheter and the passive use of a venting port to prevent procedural complications (12), the anatomic limitations of the tracheobronchial tree prevent applying this approach to the airway.
We present for the first time the efficacy and safety of SCT ablation of proximal tracheal obstructions utilizing an LMA in combination with an esophageal balloon. Use of the LMA allows two key features of a successful SCT application. First, it allows gas egress through an open ventilation system to prevent airway barotrauma. Second, it allows direct visualization throughout the cryogen application, which would be impossible for proximal tracheal lesions obscured from view by either a rigid bronchoscope or endotracheal tube.
Given the anatomical proximity of the proximal trachea to the esophagus, it is conceivable that gas may enter the gastrointestinal tract when using an LMA. Our novel use of an esophageal balloon prevents gas from rapidly entering and accumulating in the gastrointestinal tract. We also verify that there is no abdominal distention both tactilely and visually by using a team member to hold pressure on the patient’s abdomen and to monitor for abdominal distention. The importance of these safety measures is underscored by reports of severe chest pain, stricture development, and abdominal perforation during SCT of the esophagus (13).
Because our SCT application was followed by balloon tracheoplasty in both cases, it is challenging to attribute what amount of visual improvement at procedure completion was due to each therapeutic technique alone, as SCT usually has no immediately visible effect, while balloon dilation does. A recent single center retrospective analysis of SCT alone for treatment of various benign tracheal diseases did show sustained patient reported symptom improvement and objective decrease in tracheal narrowing (11). It will be important to further study SCT alone, in combination with other modalities, and in direct comparison to other techniques to further determine its near and long term effect on tracheal lesions. Our multimodal method will allow other providers without access to direct laryngoscopy (11) to play a role in this evaluation.
We believe that our novel technique accomplishes the key aspects of a successful and safe SCT application. Specifically, it allows direct airway visualization throughout cryogen application, it facilitates rapid gas egress through a ventilation system that can be easily opened, and it prevents gas egress into the closed gastrointestinal tract. Thus, we believe that this multimodal approach represents another tool to be considered by all physicians planning to apply SCT to proximal airway lesions.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patients for publication of this manuscript and any accompanying images.
References
- DiBardino DM, Lanfranco AR, Haas AR. Bronchoscopic Cryotherapy. Clinical Applications of the Cryoprobe, Cryospray, and Cryoadhesion. Ann Am Thorac Soc 2016;13:1405-15. [Crossref] [PubMed]
- Maiwand MO, Homasson JP. Cryotherapy for tracheobronchial disorders. Clin Chest Med 1995;16:427-43. [PubMed]
- Browning R, Turner JF Jr, Parrish S. Spray cryotherapy (SCT): institutional evolution of techniques and clinical practice from early experience in the treatment of malignant airway disease. J Thorac Dis 2015;7:S405-14. [PubMed]
- TruFreeze System, TruFreeze Spray Kit. Instructions for Use [Internet]. Lexington, MA: Available online: http://csamedical.com/wp-content/uploads/15-00133-06-truFreeze-Instructions-for-Use.pdf
- Ernst A, Feller-Kopman D, Becker HD, et al. Central airway obstruction. Am J Respir Crit Care Med 2004;169:1278-97. [Crossref] [PubMed]
- Sachdeva A, Pickering EM, Lee HJ. From electrocautery, balloon dilatation, neodymium-doped:yttrium-aluminum-garnet (Nd:YAG) laser to argon plasma coagulation and cryotherapy. J Thorac Dis 2015;7:S363-79. [PubMed]
- Krimsky WS, Broussard JN, Sarkar SA, et al. Bronchoscopic spray cryotherapy: assessment of safety and depth of airway injury. J Thorac Cardiovasc Surg 2010;139:781-2. [Crossref] [PubMed]
- Gage AA, Baust J. Mechanisms of tissue injury in cryosurgery. Cryobiology 1998;37:171-86. [Crossref] [PubMed]
- Finley DJ, Dycoco J, Sarkar S, et al. Airway spray cryotherapy: initial outcomes from a multi institutional registry. Ann Thorac Surg 2012;94:199-203; discussion 203-4. [Crossref] [PubMed]
- Au JT, Carson J, Monette S, et al. Spray cryotherapy is effective for bronchoscopic, endoscopic and open ablation of thoracic tissues. Interact Cardiovasc Thorac Surg 2012;15:580-4. [Crossref] [PubMed]
- Bhora FY, Ayub A, Forleiter CM, et al. Treatment of Benign Tracheal Stenosis Using Endoluminal Spray Cryotherapy. JAMA Otolaryngol Head Neck Surg 2016;142:1082-7. [Crossref] [PubMed]
- Shaheen NJ, Greenwald BD, Peery AF, et al. Safety and efficacy of endoscopic spray cryotherapy for Barrett's esophagus with high-grade dysplasia. Gastrointest Endosc 2010;71:680-5. [Crossref] [PubMed]
- Dumot JA, Vargo JJ 2nd, Falk GW, et al. An open-label, prospective trial of cryospray ablation for Barrett's esophagus high-grade dysplasia and early esophageal cancer in high-risk patients. Gastrointest Endosc 2009;70:635-44. [Crossref] [PubMed]


