Society for Translational Medicine Expert consensus on the selection of surgical approaches in the management of thoracic esophageal carcinoma
Introduction
Esophageal cancer (EC) is a highly prevalent malignancy in China. According to the data released by National Cancer Registry Center of China in 2015, the prevalence of EC was 21.17/100,000 in China in 2012, ranking fifth among all malignancies; the mortality rate was 15.58/100,000, ranking fourth. It is expected that there will be 477,900 new EC cases nationwide in 2015, whereas 375,000 people will die from EC (1,2). Worldwide, EC is the 8th most common malignancy, with 416,000 new cases in 2012 (3). At present, EC treatment is based on multidisciplinary treatment, but surgery remains the most effective therapy in optimizing long-term prognosis in patients without metastatic disease. The ideal surgical approach is a matter of debate.
Transthoracic vs. transhiatal
In the United States and in much of Europe, the transhiatal approach for esophagectomy is still common. This is typically performed through an upper laparotomy with blunt dissection of the esophagus within the mediastinum, creation of a gastric tube and celiac trunk lymphadenectomy followed by a cervical anastomosis. By avoiding a thoracic incision, the transhiatal approach has the advantage of less pulmonary complications compared to a transthoracic approach. Disadvantages are the lack of complete thoracic lymphadenectomy and the blind mediastinal dissection. Although several studies have found that a more extensive mediastinal lymphadenectomy is associated with improved survival after esophagectomy (4,5), a Dutch prospective randomized trial comparing the transhiatal versus transthoracic esophagectomy for very distal esophageal adenocarcinomas did not show a statistically significant survival advantage to one method over the other, but long term survival for Siewert type I tumors might be better with the transthoracic approach. There was not difference in survival for Siewert type II tumors between the two approaches (6). A Japanese trial (JCOG 9502) comparing left thoracoabdominal vs. transhiatal esophagogastrectomy for Siewert type II and III tumors also failed to demonstrate improved survival for a more extended lymphadenectomy (7). Minimally invasive transhiatal approach may overcome some of the limitations of the standard transhiatal esophagectomy by allowing a more extensive mediastinal lymphadenectomy, while also decreasing pulmonary complications, but an adequate mediastinal lymphadenectomy by minimally invasive transhiatal approach remains difficult. Due to its shortcomings in lymph node dissection and the risks of a blind dissection such as bleeding and tracheal injuries, the transhiatal procedure is not commonly used in China.
Impact of the left and right thoracotomy approaches for esophageal carcinoma on lymph node dissection and prognosis
The most common surgical approaches for esophageal resection include left and right thoracotomy as well as transhiatal approach. The left thoracotomy approach includes: a single left posterolateral thoracotomy; or, two incisions (a left posterolateral thoracotomy incision and a left cervical incision). The right thoracotomy approaches include two approaches (midline laparotomy and right posterolateral thoracotomy, Ivor Lewis; or midline laparotomy and right posterior muscle-sparing thoracotomy), or three incisions (left neck incision + right posterolateral thoracotomy incision + midline abdominal incision, McKeown). Both right sided approaches can be performed by minimally invasive techniques. In recent years, along with advances in endoscopic technologies, the thoracoscopic/laparoscopic esophagectomy + left cervical anastomosis has gradually been popularized in China and has become a mainstream procedure in large hospitals.
There is a dense lymphatic network in the esophageal wall. Once EC involves the submucosal layer, lymph node metastasis occurs in over 20% of patients (8). The risk of lymph node metastasis is associated with depth of tumor invasion (T stage). The number of metastatic lymph nodes negatively correlates with survival. Studies have shown that the number of harvested lymph nodes may impact survival (4,5). Liu et al. (9) and Chen et al. (10) from Fujian Tumor Hospital reported that the number of involved lymph nodes after three-field lymph node dissection in thoracic esophageal carcinoma was an important independent prognostic factor; according to the 7th Edition of the AJCC Cancer Staging Manual, the 5-year survival in EC with N0, N1, N2, or N3 metastasis was 77.5%, 41.2%, 22.2%, and 7.0%, respectively (P<0.0001) .Therefore, selecting the corresponding surgical approach according to the specific characteristics of EC and its stage and carrying out standardized and complete lymph node dissection are critically important for improving survival in patients with EC and lowering the risk of post-operative recurrence (9-15).
In China, the left thoracotomy approach was the first approach used for treatment of EC, and it is still widely applied in northern China. During the treatment of EC via the left thoracotomy approach, the presence of the aortic arch and the left subclavian artery and the narrow triangle area above the arch limits the ability to dissect the superior mediastinum lymph nodes, especially those near the left and right tracheoesophageal areas and recurrent laryngeal nerves. Resection of lymph nodes in the celiac axis may also be limited. Complete thoraco-abdominal lymph node dissection therefore cannot be achieved completely via a left side approach. Besides, the use of multimodality therapy (neoadjuvant chemo/radiation therapy followed by surgery) was not common in China prior to 2000. As a result, the effectiveness of surgical treatment for EC had not been improved before 2000 and the patients’ 5-year survival remained around 30% (12,16-18).
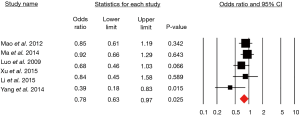
In the last 10 years, many hospitals in China, especially those in southern China, have adopted procedures such as the Ivor Lewis esophagectomy (a midline laparotomy plus right posterolateral thoracotomy or right posterior muscle-sparing thoracotomy), during which the lymph nodes in the thoracic and abdominal fields (especially those near the left and right tracheoesophageal areas and recurrent laryngeal nerve) were completely removed; owing to this reason partly, the patients’ 5-year survival was remarkably improved (19-21). In addition, some hospitals in China also have used radical en bloc resection of esophagus, including three field lymph node dissection (cervical, thoracic, and abdominal fields); en bloc three field esophagectomy allows for a thorough removal of lymph nodes in superior mediastinum and neck and may improve survival (22,23). These results of three-field lymph node dissection were consistent with the findings by Japanese investigators and others who are more experienced in three-field lymph node dissection for EC (12,14,24). Unfortunately, postoperative complications are significantly increased with the en bloc approach (12,14,24,25). Currently, the 5-years overall survival range 38.7–57.6% following surgical treatment of esophageal carcinoma via the right thoracotomy approach, showing superiority to the left thoracotomy approach (19,21,26-28). Mao et al. (21) from Chinese Academy of Medical Sciences reported that they had performed surgical treatment in 559 patients with thoracic EC and the average number of lymph nodes dissected via the left and right thoracotomy approaches was 23.4 and 24.6, respectively (P<0.001) and the postoperative 5-year survival was 38.2% and 42.1%, and the differences were not statistically significant (χ2=0.246, P=0.620). In a retrospective controlled study comparing two approaches: the left thoracotomy approach and right thoracotomy approach, Luo et al. (27) from Sun Yat-sen University Cancer Center found that the average number of lymph node dissected was 11.8±6.6 in left thoracotomy approach group and 16.3+8.0 in right thoracotomy approach group (P<0.001). The 1- and 3-year survivals were 78.9% and 48.2% in the left thoracotomy approach group and 82.6% and 57.6% in the right thoracotomy approach group respectively. The median survival in the left and right thoracotomy groups was (25.63±0.63) months and (27.42±1.01) months, respectively, showing no significant difference (P=0.080); the incidence of complications in the left and right groups was 35.4% and 57.6% (P<0.001). The recurrence rate in the left and right groups was 50.0% (175/350) and 42.4% (56/132) (P=0.138). It was concluded that although the left thoracotomy approach was easy to perform, reducing postoperative complications, while right thoracotomy approach had an increased number of lymph nodes dissected. In a clinical randomized controlled study performed by Li et al. (29) from Fudan University Shanghai Cancer Center, the average number of lymph node dissected via the left and right thoracotomy approach was 18 and 22 (P<0.001) and the incidence of postoperative complications was 41.3% and 30% (P=0.04) respectively; they concluded that the procedure via the right thoracotomy approach not only could remove more lymph nodes (compared with left thoracotomy approach) but also could lower the incidences of postoperative complications; however, the authors did not describe data on long-term survival. Peng et al. (19) from Sichuan provincial Cancer Hospital performed Ivor Lewis esophagectomy with two-field (thoraco-abdominal) lymph node dissection via median laparotomy and right posterolateral thoracotomy approach, and obtained a postoperative 5-year survival of 55.49%. Nasser Altorki et al. (24) from U.S.A. reported an average yield of 47 lymph nodes obtained by a 3-field lymphadenectomy via the right thoracotomy with a postoperative 5-year survival of 51%. Shimada et al., (12) from Japan reported a post-operative 5-year survival of 52.0% after radical treatment for EC via the right thoracotomy approach. A summary of recent studies on the results of lymph node dissection and prognosis following surgical treatment of EC after dissection of two or three-filed lymphadenectomy via left thoracotomy approach is depicted in Table 1. The currently available findings both in China and abroad have shown that the right thoracotomy approach obtains a more extensive lymphadenectomy (in thorax and abdomen) than the left thoracotomy approach and the right thoracotomy approach enables a 3-field lymph node dissection (not possible via left thoracotomy) and in this may improve prognosis in selected patients (30); However, most of the studies were retrospective, and there are few prospective randomized studies with large sample size. Therefore, large-scale prospective randomized controlled studies are still warranted to confirm the impact of surgical approach on survival in patients with EC.
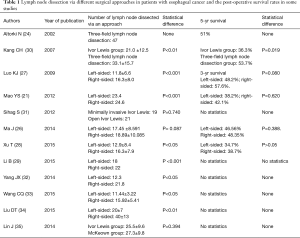
Full table
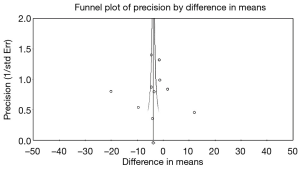
A meta-analysis by Guy Eslick of these studies which includes 3,539 patients (2,080 esophagectomy through left thoracotomy); and 1,459 patients resected via the right thoracotomy) and compared the number of lymph nodes retrieved. Survival analysis was possible in only 1,019 left-sided esophagectomy and 920 right-sided esophagectomy patients. The pooled analysis suggests that the left-sided approach retrieves 3.54 less lymph nodes than the right-sided approach (Figure 1) (36).

There was a high level of heterogeneity between the studies (I2=96.65, P<0.001), but there was no evidence of publication bias (P=0.99) (Figure 2).
Moreover, the right-sided approach offers superior survival compared to the left-sided approach (Figure 3).
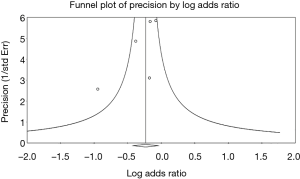
These studies had low heterogeneity that was not statistically significant and again there was no evidence of publication bias (Figure 4).
Minimally invasive approaches
Minimally invasive esophagectomy (MIE) may include different approaches such as total MIE (laparoscopy, and thoracoscopy in different positions (in lateral position, in prone position or in semiprone position), the RAMIE (robot assisted MIE), the MIE transhiatal approach and hybrid approaches (type 1 combines laparoscopy and right thoracotomy and type 2 include the laparotomy and thoracoscopy). MIE means less operative trauma and as consequence of this has potential advantages such as less blood loss, less postoperative respiratory infections, less pain, shorten intensive care and hospital stay, and better postoperative quality of life (36). Uncertainty includes questions about increased operative time, and the long-term oncologic safety and outcomes. Multiple studies, long series, meta-analysis and two randomized control trials, have demonstrated the short and long-term oncological safety (at three years follow up) of MIE compared to open esophagectomy (36-39) The TIME trial, a small, prospective trial (115 patients in two arms) found significantly decreased pulmonary infections in patients undergoing MIE compared to open esophagectomy (36). In addition, quality of life at one-year follow-up was better in the MIE group. Although it was a small study, no difference in survival at one and three years was seen (40,41). Many centers in China have had large experiences with MIE with acceptable short-term outcomes. Meta-analysis of Yibulayin et al. (42) included 57 studies with 15,790 patients. They concluded that MIE is superior to OE in terms of perioperative complications (also anastomotic leaks) and in hospital mortality. Mu et al. (43), performed a controlled randomized study comparing the short-term outcomes and 3-year survival between total MIE 3 stage McKeown and the so called dual-incision esophagectomy through an open left thoracotomy (DIE = Sweet procedure). They concluded that MIE and DIE yielded comparable short-term outcomes, however MIE was associated with better three year overall and disease-free survival (43). Moreover, Sun et al. (44) performed an important control randomized study comparing two groups of patients operated by MIE 3 stage Mckeown esophagectomy. One group was treated by early oral feeding and the other by traditional late oral feeding. They concluded that between the two forms of oral feeding (early versus late oral feeding after MIE McKeown) there were no differences in major complication rates and importantly there was an increase in Quality of Life if early feeding was given. Likewise, Wang et al. from Fudan University Shanghai Cancer Center performed a propensity-score matched analysis of 735 MIE patients compared to 652 open esophagectomy patients and found improved quality of life among the MIE patients. The MIE group also had lower rates of complication. No difference in survival was found (45). Using the National Cancer Database, Weksler did not find a significant difference in survival among patients undergoing open, minimally invasive, or robotic esophagectomy (46).
How to select approaches of individualized surgical treatment for EC at different sites and stages
Based on currently available guidelines and expert consensus in China, we suggest a few recommendations for patient selection. For resectable middle and lower thoracic esophageal carcinoma with if preoperative EUS, and CT scan of the chest and abdomen, and whole body PET-CT reveals a clinical stage of T2N0-1M0, neoadjuvant concurrent chemo radiation followed by resection of esophageal carcinoma with thoracic or cervical anastomosis via the conventional two incisions (abdomen and chest) or three incisions (chest, abdomen, and neck), minimally invasive or open with complete two-field (thorax and abdomen) or three-field (neck, thorax, and abdomen) lymphadenectomy is recommended. Patients with clinical stage of T1bN0 can forgo preoperative therapy. For resectable upper thoracic EC, Akiyama et al. (47) found that the upper thoracic EC might have a high probability of metastasis in the upper mediastinum and in the cervical areas and therefore the three-field lymphadenectomy should be considered. The rates of cervical lymph node metastasis of the upper, middle, and lower EC were 46.3%, 29.2%, and 27.2%, respectively. According to Liu et al. (9), the three-field lymphadenectomy (compared with two-field lymphadenectomy) can significantly prolong the postoperative 5-year survival in patients with upper thoracic EC (53.2% vs. 34.1%, P=0.002). Therefore, for resectable upper thoracic EC, esophageal resection and left cervical esophagogastrostomy via 3 incision procedure, MIE, total or hybrid, or open, with total two-field (thorax and abdomen) or three-field (neck, thorax, and abdomen) lymphadenectomy is recommended. For these patients, whether a left or right thoracotomy approach should be selected for surgical treatment remains controversial (21,26-28). According to Ma Ma et al. (26) from Shanghai Zhongshan Hospital, for patients without suspected lymph node metastasis in the superior mediastinum, the 5-year survival rate following left thoracotomy and right thoracotomy was not different, 46.56% and 48.35%, respectively (P=0.388), and the median survival was 52 months and 48 months; the incidence of postoperative complications in the left and right thoracotomy groups was 12.3% and 20.4% (P=0.002), the local relapse rate was 14.8% and 13.3% (P=0.695), and the distant relapse rate 30.4% and 24.8% (P=0.274) respectively. In our opinion, for patients without suspected lymph node metastasis in the superior mediastinum, there are until now no clear evidence of statistical difference in terms of local relapse and long-term survival between left and right thoracotomy approach. However, for middle and lower thoracic EC, if the preoperative EUS + thoraco-abdominal CT/PET-CT reveals the presence of suspected lymph node involvement and metastasis in the superior mediastinum, the current recommended treatment is: resection of EC and complete two-filed/three-field lymph node dissection via the conventional two or three stage, MIE, total or hybrid, or open through the right thoracic approach including a two or three-field lymphadenectomy (18-20,40).
Role of neoadjuvant therapy
Important point in the treatment of EC is the current implementation of neoadjuvant therapy in the form of chemoradiotherapy or chemotherapy (48,49). Remarkable are the long-term results of the CROSS study, in which patients with clinically resectable esophageal or junctional cancer (cT-1N1M0 or cT2-3N0-1M0) were randomly assigned to receive weekly administration of Carboplatin and Paclitaxel for 5 weeks with concurrent radiotherapy (41.4 Gy given in 23 fractions, 5 days per week), followed by surgery, or surgery alone. Radical resection (R0) was accomplished in 92% of patients in the group of nCRT plus surgery group and 69% in the surgery alone group. Because the decrease in size of the tumors after nCRT, resection was considered easier to perform. Moreover, a pathological complete response was achieved in 49% of the squamous cell cancer patients. Five-year overall survival rate was 47% in the nCRT plus surgery group and 33% in the surgery alone group, respectively (in the Squamous cell cancer it was, 61% versus 30% respectively).
Moreover, the neoadjuvant chemotherapy studies, such as the MAGIC trial have showed a 5 years improvement of 13% compare to surgery alone (48). Important is to remark, that the highest benefit of the CROSS neoadjuvant chemoradiotherapy is observed in the squamous cell cancer which is radiosensitive and the most frequent EC in China.
Levels of evidence
According to the above systematic review, the levels of evidence were at level III, the quality of the evidence was average, and the strength of recommendation is medium. Thus, according to the findings of retrospective studies and a few prospective studies, performed in China and abroad, the right transthoracic approach is superior to left thoracotomy approach for performing the two-field lymphadenectomy for thoracic EC. This approach can reduce the incidence of postoperative local relapse in the upper mediastinum and thus increase the survival benefit. Therefore, for resectable upper thoracic EC, EC resection plus two-field (thorax and abdomen) or three-field (neck, thorax, and abdomen) lymphadenectomy dissection is strongly recommended through the right thoracotomy approach. However, due to the low evidence levels, large-scale prospective randomized controlled studies are still warranted.
Future research directions
More research is needed to better definitively evaluate if a left thoracotomy approach yields equivalent outcomes to a right thoracotomy approach for middle and lower thoracic EC without suspected lymph node involvement.
Acknowledgements
The authors would like to express sincere appreciation to Prof. Wayne L. Hofstetter (Department of Thoracic and Cardiovascular Surgery, University of Texas, MD Anderson Cancer Center, Houston, Texas, USA) for his valuable suggestions to improve the manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Chen WQ, Zheng RS, Zhang SW, et al. Report of cancer incidence and mortality in China, 2012. China Cancer 2016;25:1-8.
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Peyre CG, Hagen JA, DeMeester SR, et al. Predicting systemic disease in patients with esophageal cancer after esophagectomy: a multinational study on the significance of the number of involved lymph nodes. Ann Surg 2008;248:979-85. [Crossref] [PubMed]
- Peyre CG, Hagen JA, DeMeester SR, et al. The number of lymph nodes removed predicts survival in esophageal cancer: an international study on the impact of extent of surgical resection. Ann Surg 2008;248:549-56. [PubMed]
- Omloo JM, Lagarde SM, Hulscher JB, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the mid/distal esophagus: five-year survival of a randomized clinical trial. Ann Surg 2007;246:992-1000; discussion 1000-1. [Crossref] [PubMed]
- Kurokawa Y, Sasako M, Sano T, et al. Ten-year follow-up results of a randomized clinical trial comparing left thoracoabdominal and abdominal transhiatal approaches to total gastrectomy for adenocarcinoma of the oesophagogastric junction or gastric cardia. Br J Surg 2015;102:341-8. [Crossref] [PubMed]
- Ancona E, Rampado S, Cassaro M, et al. Prediction of lymph node status in superficial esophageal carcinoma. Ann Surg Oncol 2008;15:3278-88. [Crossref] [PubMed]
- Liu SY, Zhu KS, Zheng QF, et al. Comparison of survival between three-field and two-field lymph node dissections for thoracic esophageal squamous carcinoma. Chin J Thorac Cardiovasc Surg 2014;30:645-8.
- Chen YM, Li JQ, Zhu KS, et al. The relationship between number of metastatic lymph node and prognosis of thoracic-esophageal cancer patients treated with radical resection. Chin J Thorac Cardiovasc Surg 2014;30:76-8.
- Hagen JA, DeMeester SR, Peters JH, et al. Curative resection for esophageal adenocarcinoma: analysis of 100 en bloc esophagectomies. Ann Surg 2001;234:520-30; discussion 530-1. [Crossref] [PubMed]
- Shimada H, Okazumi S, Matsubara H, et al. Impact of the number and extent of positive lymph nodes in 200 patients with thoracic esophageal squamous cell carcinoma after three-field lymph node dissection. World J Surg 2006;30:1441-9. [Crossref] [PubMed]
- Kayani B, Zacharakis E, Ahmed K, et al. Lymph node metastases and prognosis in oesophageal carcinoma--a systematic review. Eur J Surg Oncol 2011;37:747-53. [Crossref] [PubMed]
- Natsugoe S, Matsumoto M, Okumura H, et al. Clinical course and outcome after esophagectomy with three-field lymphadenectomy in esophageal cancer. Langenbecks Arch Surg 2010;395:341-6. [Crossref] [PubMed]
- Xiao ZF, Yang ZY, Wang LH, et al. Influence of the number of lymph node metastasis on survival and significance of postoperative radiotherapy for esophageal carcinoma. Zhonghua Zhong Liu Za Zhi 2004;26:112-5. [PubMed]
- Mao Y, He J, Gao S, Xue Q. Controversies in the surgical treatment for esophageal carcinoma and future investigation. Zhonghua Wei Chang Wai Ke Za Zhi 2015;18:851-4. [PubMed]
- Mao YS, He J, Cheng GY. Surgical treatment of esophageal neoplasms in China: status quo and future development. Chin J Oncol 2010;32:401-4.
- He J. Guidelines on the standardized management of esophageal cancer. Beijing: Peking Union Medical College Press, 2013.
- Peng L, Chen LH, Li Q, et al. Ivor Lewis esophageal subtotal resection and two-field lymph node dissection and their impacts on the prognosis. China Oncology 2003;13:574-6.
- Wu CR, Xue HC, Zhu ZH, et al. Analysis of the therapeutic effect of esophagectomy with extended 2-field lymph node dissection for esophageal carcinoma. Zhonghua Zhong Liu Za Zhi 2009;31:630-3. [PubMed]
- Mao YS, He J, Dong JS, et al. Comparison of the results of lymph node dissection via left versus right thoracotomy. Zhonghua Zhong Liu Za Zhi 2012;34:296-300. [PubMed]
- Wu N, Chen ZM, Pang LW, et al. Clinical analysis of lymphadenectomy in patients with esophageal carcinoma underwent single left thoracal incision and cervico-right thoracic-abdominal triple incision. Cancer Research and Clinic 2013;25:77-9.
- Zhang YW, Hu H, Miao LS, et al. Comparison of 2-field lymphadenectomy with 3-field lymphadenectomy formiddle third thoracic esophageal carcinoma. China Oncology 2008;18:537-41.
- Altorki N, Kent M, Ferrara C, Port J. Three-field lymph node dissection for squamous cell and adenocarcinoma of the esophagus. Ann Surg 2002;236:177-83. [Crossref] [PubMed]
- Ding JY, Xu ST. Three-field lymph node dissection for esophagal cancer: review and prospect. Chinese Journal of Clinical Medicine 2011;18:879-82.
- Ma J, Zhan C, Wang L, et al. The sweet approach is still worthwhile in modern esophagectomy. Ann Thorac Surg 2014;97:1728-33. [Crossref] [PubMed]
- Luo KJ, Fu JH, Hu Y, et al. Efficacy of surgical resection of left and right transthoracic approaches for middle thoracic esophageal squamous cell carcinoma. Ai Zheng 2009;28:1260-4. [Crossref] [PubMed]
- Xu T. Comparison of the modified Ivor-lewis procedure versus conventional left thoracotomy approach left posterolateral thoracotomy in the treatment of esophageal squamous cell carcinoma. Journal of Clinical Medicine in Practice 2015;19:86-7,96.
- Li B, Xiang J, Zhang Y, et al. Comparison of Ivor-Lewis vs Sweet esophagectomy for esophageal squamous cell carcinoma: a randomized clinical trial. JAMA Surg 2015;150:292-8. [Crossref] [PubMed]
- Kang CH, Kim YT, Jeon SH, et al. Lymphadenectomy extent is closely related to long-term survival in esophageal cancer. Eur J Cardiothorac Surg 2007;31:154-60. [Crossref] [PubMed]
- Sihag S, Wright CD, Wain JC, et al. Comparison of perioperative outcomes following open versus minimally invasive Ivor Lewis oesophagectomy at a single, high-volume centre. Eur J Cardiothorac Surg 2012;42:430-7. [Crossref] [PubMed]
- Yang JX, Chen XF, Wang ZW, et al. Efficacy of different ways of left and right transthoracotomy approaches on patients with middle thoracic esophageal cancer. China Modern Doctor 2014;27:5-7.
- Wang CQ. The clinical effect comparison of modified Ivor-Lewis surgical and left thoracotomy surgery in treatment of lower esophageal cancer. China Modern Doctor 2015;53:33-4,37.
- Liu DT, Chen SB, Li H, et al. Comparison of short-term effect of left and right transthoracotomy approach surgery treating thoracic esophageal squamous cell carcinoma. China Medicine 2015;10:546-8.
- Lin J, Kang M, Lin J, et al. Short-term efficacy comparison between Ivor-Lewis approach and McKeown approach in minimally invasive esophagectomy. Zhonghua Wei Chang Wai Ke Za Zhi 2014;17:888-91. [PubMed]
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [Crossref] [PubMed]
- Palanivelu C, Prakash A, Senthilkumar R, et al. Minimally invasive esophagectomy: thoracoscopic mobilization of the esophagus and mediastinal lymphadenectomy in prone position--experience of 130 patients. J Am Coll Surg 2006;203:7-16. [Crossref] [PubMed]
- Sihag S, Wright CD, Wain JC, et al. Comparison of perioperative outcomes following open versus minimally invasive Ivor Lewis oesophagectomy at a single, high-volume centre. Eur J Cardiothorac Surg 2012;42:430-7. [Crossref] [PubMed]
- Biere SS, Cuesta MA, van der Peet DL. Minimally invasive versus open esophagectomy for cancer: a systematic review and meta-analysis. Minerva Chir 2009;64:121-33. [PubMed]
- Maas KW, Cuesta MA, van Berge Henegouwen MI, et al. Quality of Life and Late Complications After Minimally Invasive Compared to Open Esophagectomy: Results of a Randomized Trial. World J Surg 2015;39:1986-93. [Crossref] [PubMed]
- Straatman J, van der Wielen N, Cuesta MA, et al. Minimally Invasive Versus Open Esophageal Resection: Three-year Follow-up of the Previously Reported Randomized Controlled Trial: the TIME Trial. Ann Surg 2017;266:232-236. [Crossref] [PubMed]
- Yibulayin W, Abulizi S, Lv H, et al. Minimally invasive oesophagectomy versus open esophagectomy for resectable esophageal cancer: a meta-analysis. World J Surg Oncol 2016;14:304. [Crossref] [PubMed]
- Mu JW, Gao SG, Xue Q, et al. Comparison of short-term outcomes and three yearsurvival between total minimally invasive McKeown and dual-incision esophagectomy. Thorac Cancer 2017;8:80-7. [Crossref] [PubMed]
- Sun HB, Li Y, Liu XB, et al. Early Oral Feeding Following McKeown Minimally Invasive Esophagectomy: An Open-label, Randomized, Controlled, Noninferiority Trial. Ann Surg 2018;267:435-42. [Crossref] [PubMed]
- Wang H, Shen Y, Feng M, et al. Outcomes, quality of life, and survival after esophagectomy for squamous cell carcinoma: A propensity score-matched comparison of operative approaches. J Thorac Cardiovasc Surg 2015;149:1006-14; discussion 1014-5.e4.
- Weksler B, Sullivan JL. Survival After Esophagectomy: A Propensity-Matched Study of Different Surgical Approaches. Ann Thorac Surg 2017;104:1138-46. [Crossref] [PubMed]
- Akiyama H, Tsurumaru M, Udagawa H, et al. Radical lymph node dissection for cancer of the thoracic esophagus. Ann Surg 1994;220:364-72; discussion 372-3. [Crossref] [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Shapiro J, van Lanschot JJB, Hulshof MCCM, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]