
Electromagnetic navigation bronchoscopy: the initial experience in Hong Kong
Introduction
Pulmonary nodule (PN) is becoming an increasing diagnostic challenge. PNs are increasingly detected by the widespread availability of computed tomography (CT). There are many differential diagnoses of PN, the most sinister is malignancy; infections [particularly tuberculosis (TB)], vasculitis, benign tumours, etc. are other possible diagnosis. The prevalence of PNs appears to be much higher in the Asian population versus that of Europe and North America (1). In addition, the evaluation of PNs in Asia is complicated by the high prevalence of TB and the fact that non-smoking history does not have any weight in discounting malignancy risk (2). Therefore, an Asian consensus guideline suggests less reliance on PET scanning, surgical diagnosis or surveillance, and it is in favour of more non-surgical biopsies [e.g., CT-guided transthoracic needle aspiration (CT-TTNA) or guided bronchoscopy] (3). CC-TTNA has a high diagnostic yield (~90%); however, it carries a relatively high risk of iatrogenic pneumothorax (up to 25%) (4,5).
Several guided bronchoscopic methods are available to diagnose PN, including electromagnetic navigation bronchoscopy (ENB), radial endobronchoscopic ultrasonography (R-EBUS) and virtual bronchoscopy (VB). Collectively they have better diagnostic yield (pooled diagnostic yield ~70%) over traditional flexible bronchoscopy (FB)/transbronchial biopsy (TBBx) and lower complication rate over CT-TTBA (6). ENB is gaining acceptance and popularity amongst bronchoscopists as one of the tools to diagnose PN. It can be used on its own, or be combined with R-EBUS to enhance the diagnostic yield (7,8). Its diagnostic yield and safety have been demonstrated in two meta-analyses (9,10). A pioneering work in Asia also demonstrated good sensitivity (92.9%) for small PNs (5.0–30.0 mm) (11).
Studies included in the previous meta-analyses studied lesions outside the field of conventional flexible bronchoscope (FB), the majority of them did not use other guiding tools (9,10). In the pioneering work of Asia, case selection was based on a case conference, and a bronchus sign was a prerequisite for ENB (11).
The authors’ department routinely uses FB in combination with R-EBUS and fluoroscopy for evaluation of PNs. Our department has formally installed the first ENB system in Hong Kong after an evaluation period. We report our initial experience of ENB in diagnosing PNs for which FB using fluoroscopy and R-EBUS has failed or is perceived to have very low yield. These probably represented more difficult lesions to be biopsied when compared to previous studies (9-11).
Methods
This is a retrospective study of the diagnostic yield and complication rate of ENB performed in the Department of Medicine & Geriatrics, United Christian Hospital, Hong Kong, from April 2015 to June 2016. The choice of study period (and hence sample size) was to allow early assessment of this new technology. Patients were followed up for at least 1 year at the time of analysis to evaluate the final diagnosis. The study was approved by the Research Ethics Committee (Kowloon Central/Kowloon East Region), Hong Kong. Consent from the patient was waived. Standards for Reporting Diagnostic accuracy studies (STARD 2015) was used for reporting.
Settings
United Christian Hospital is an acute hospital in the East Kowloon region of Hong Kong with a catchment population of over 1 million. The Division of Respiratory Medicine receives unselected referrals from emergency department, general practitioners, community physicians, other medical teams and other departments in the hospital.
Patient selection
Consecutive patients detected to have PNs on CT scan during the study period were discussed in regular multi-disciplinary team (MDT) meetings comprising of respiratory physicians, thoracic surgeons, radiologists and oncologists. If a histological diagnosis was deemed necessary and FB was the preferred modality over CT-TTNA, the patients were offered ENB, if they met the following criteria: (I) they fulfilled the standard indication to undergo FB (12), have signed a written informed consent, and without known contraindication (e.g., unstable haemodynamics, severe hypoxaemia, uncorrectable coagulopathy); in addition, (II) they have failed a conventional FB guided by fluoroscopy and R-EBUS to reach the target PN; or (III) conventional FB guided by fluoroscopy and R-EBUS was highly likely to fail: (i) target PN was not visible on plain chest X-ray (and hence fluoroscopy), by virtue of its small size and/or position (e.g., obscured by bone, hilar structures, heart, diaphragm); or (ii) target PN was a ground glass opacity on CT (unlikely to be visualised by fluoroscopy and R-RBUS); or (iii) target PN was a lesion amongst/within lesions (Figure 1), e.g., target PN is one of many PNs overlapping each other on X-ray, target PN is behind an old TB scar, the target is a small PET positive area within the scar of a previously irradiated lung tumour.
Patients should not have the following ENB-specific contraindications: (I) has a permanent pacemaker in situ; or (II) pregnant; or (III) paediatric age group (equipment not approved for paediatric age group and not calibrated for smaller body size).
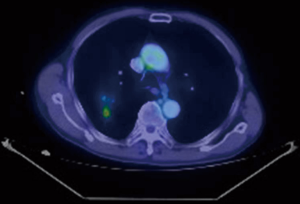
ENB
ENB was performed under intravenous sedation and local anaesthesia (13). Excellent review is available that describes the techniques of ENB performance in details (14). Briefly, CT thorax images of the patient is analysed by dedicated ENB software. The target lesion is identified, and pathways to the lesion via the bronchial tree are planned semi-automatically with the planning software. Once planning is complete, a 3-dimensional reconstructed view of the bronchial tree with planned route(s) to the lesion is generated by the ENB software. During the ENB procedure, a location board that emits electromagnetic waves is placed on the patient’s body. A sensing device is then inserted into the working channel of the bronchoscope, and the bronchoscope is advanced into various part of the bronchial tree to allow automatic matching of the CT images with the real-life anatomy of the patient (registration). Once registration is satisfactory, navigation can proceed. The bronchoscope is guided by the sensing device to reach the lesion (analogous to driving with Global Position System guidance), using pre-planned pathway(s) within VB images that are displayed side-by-side with the actual bronchoscopic images. When the tip of the sensing device is aligned with, and in close proximity to the virtual target, the authors routinely confirm navigation success with R-EBUS (7) and fluoroscopy before sampling. Sampling methods includes transbronchial needle aspiration, TBBx, brush cytology and bronchoalveolar lavage of the target area with catheter suction technique (15).
Data collection
The following data is retrieved from the case notes for each case: patient’s demographics, lesion characteristics [maximum diameter, smallest distance to pleura from the lesion periphery, presence of a bronchus sign (16)], doses of sedative and local anaesthetic used, procedure time, diagnosis obtained from ENB and complications.
Patients were followed up for at least 1 year to obtain a final diagnosis if ENB had not been diagnostic. Additional procedures (e.g., CT-TTNA, surgical biopsy) were arranged if deemed appropriate, and if patients consented. Lesions that resolved (completely or >50% diameter) during the follow up period were classified as benign.
A positive diagnosis by ENB was defined if a specific diagnosis was made with histological, cytological or microbiological samples obtained from ENB (e.g., carcinoma, TB) without the need for additional diagnostic procedure. False positive is assumed to be absent. A negative result by ENB was defined if no specific diagnosis was made with ENB, and additional biopsy procedures were necessary to obtain a diagnosis. False negative result was defined as no ENB diagnosis but further biopsy yielded a positive diagnosis or there was radiological progression of CT thorax compatible with lung malignancy. True negative was defined as no ENB diagnosis and surgical exploration did not yield any pathology and/or follow up CT showed complete or partial (>50% diameter) resolution of the lesion.
Statistical analyses
Summary statistics are expressed as frequencies (%), mean [standard deviation (SD)] or median [interquartile range (IQR)] where appropriate. Comparisons of numerical data between independent groups were performed with Student’s t-test or Mann-Whitney test, pairs of categorical data were analysed with Chi-squared test or Fisher’s exact test, where appropriate. A two-tailed P value of <0.05 was considered statistically significant. Statistical analyses were performed using SPSS for Windows, version 16.0 (SPSS Inc., Chicago, IL, USA).
The diagnostic yield including sensitivity [true positives/(true positives + false negatives)], specificity [true negative/(false positive + true negative)], negative predictive value [NPV, true negatives/(true negatives + false negatives)], and accuracy [(true positives + true negatives)/total] were calculated. Since false positive is assumed to be absent, specificity is by definition 100%.
Results
During the study period, 110 patients were referred for consideration of ENB. Eleven did not proceed to ENB (5 had poor quality CT for pathway planning, 3 failed registration before ENB, 2 had central airway lesion thus ENB not necessary, 1 had sputum culture confirming TB while awaiting ENB). Ninety-nine patients underwent ENB (59 had PNs not visible on fluoroscopy, 26 had failed conventional FB with R-EBUS/Fluoroscopic guidance, 14 had target lesions amongst/within lesions) (Table 1, Figure 2).
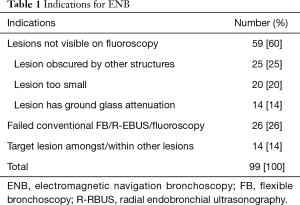
Full table
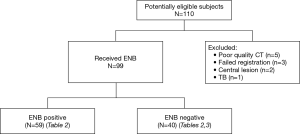
The mean (SD) age was 69.1 (11.4) with 73 males (73.7%). The median size of the target PN was 2.6 cm (IQR 2.0–3.7 cm). The median smallest distance of the PN to the pleura was 0.94 cm (IQR 0.45–1.90 cm). The PNs are located in the lower lobes in 26 patients (26.3%). Bronchus sign was present in 83 (84%), 50 centrals (50.5%), and 33 adjacent to (33.3%) the PN. The mean (SD) procedure time was 57 (18.5) minutes. The mean (SD) doses of intravenous pethidine, diazepam and topical lignocaine was 75.4 (18.5), 8.3 (4.0) and 292 (73.5) mg respectively.
An overall of 87 patients had non-resolved CT lesions and had specific pathologies identified (87.9%). The total number of malignant PNs was 67 (67.7%) in the entire cohort (50 diagnosed by ENB), TB accounted for 14 PNs (14.1%, 7 diagnosed by ENB), organising pneumonia 4 PNs (4%, 2 diagnosed by ENB), and benign tumours in 2 PNs (2%, all diagnosed by surgical resection).
ENB yielded a specific diagnosis (true positives) in 59 cases (59.6%), 49 had primary lung cancers (49.5%), 7 had TB (7.1%); other diagnoses are listed in Table 2. Twelve cases had negative ENB results and follow up CT showed resolution of the lesions (12.1%), they are regarded as true negatives. True positive cases were older [mean age 71.3 (9.5)] when compared to ENB negative cases [mean age 65.9 (13.3)] (P=0.034). The diameter of ENB positive cases were larger [median 2.9, IRQ 2.3–4.0 cm] vs. negative cases [median 2.2, IQR 1.6–3.5 cm] (P=0.007). No relationship was found between ENB positive and negative cases with respect to gender, lobe harbouring the PN or presence of bronchus sign. TBBx using biopsy forceps (n=92) was associated with a true positive diagnosis in 63.0% vs. 14.3% when it was not done (P=0.016). Catheter suction technique (n=31) was associated with a true positive diagnosis in 77.4% vs. 49.2% when it was not used (P=0.014).
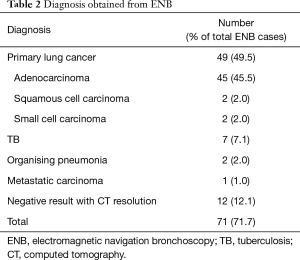
Full table
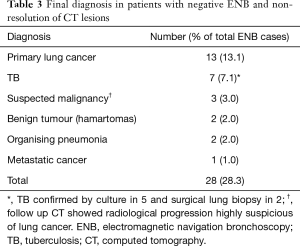
In 28 cases, ENB was negative (28.3%) and follow-up CT showed non-resolution of PNs. Further diagnostic evaluation included surgical lung biopsies in 18 (18.2%) and CT-TTNA in 2 (2.0%). Eight patient declined invasive investigations (8.1%), 5 of them had subsequent microbiological confirmation of TB (5.1%), 3 only agreed for CT surveillance (3.0%) which showed progression of PNs suspicious of malignancy. The final diagnoses are listed in Table 3. They are regarded as false negative ENB.
Full table
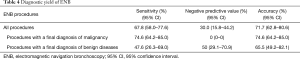
The overall accuracy by ENB was 71.7% [(59+12)/99], sensitivity 67.8% [59/(59+28)], specificity 100% [12/(0+12)] and NPV 30% [12/(28+12)]. For the subgroup of malignant PNs (including 3 highly suspicious cases) as final diagnosis, the accuracy was 74.6% [50/(50+17)]. For benign diseases, the accuracy was 65.6% [(9+12)/32] (Table 4).
Full table
Complications occurred in 3 patients: 1 had a pneumothorax requiring chest tube insertion for drainage (1.0%), 1 had post-TBBx bleeding of ~200 mL requiring intubation for airway protection for 3 days (1.0%), 1 had severe chronic obstructive pulmonary disease (COPD) and developed post-bronchoscopy respiratory failure requiring mechanical ventilation for 2 days (1.0%). No patient died as a result of ENB or its complications.
Discussion
The current study suggests that the prevalence of serious diseases including malignancy (67.7%) and TB (14.1%) is high in PNs seen in a typical referral centre in Asia. This finding lends support to an Asian consensus guideline on the evaluation of PN in favour of more reliance on biopsy to obtain a diagnosis, over observation or PET scan in the evaluation of PNs in the local settings (3).
Although it is not a randomised controlled study with direct comparison of various guided bronchoscopic methods to diagnose PNs, the current study requires patients to have failed conventional bronchoscopy with R-EBUS/fluoroscopy (or have a high likelihood of failure by virtue of the lesion characteristics as agreed in an MDT meeting) before ENB is considered. The diagnostic yield with conventional FB/R-EBUS/fluoroscopy in this group of patients is expected to be minimal. The current cohort of patients is generally not candidate for CT-TTNA after MDT discussion (e.g., severe COPD/emphysema, needle path obstructed by bone, deep seated lesion, proximity to vital structures, patient’s preference). Indeed, for ENB negative cases, the majority required surgical biopsy to obtain a diagnosis, and CT-TTNA was judged suitable in only 2 instances. Therefore, the overall accuracy (71.7%) and sensitivity (67.8%) of ENB and low rates of complications are considered welcomed additional values over the commonly used alternative biopsy methods used in Hong Kong and Asian countries. Although the current study did not compare VB with ENB, the software inherent in the ENB system can be easily adapted to allow VB and bronchoscopic sampling with an ultra-thin bronchoscope (UTB) (13), thus providing an option of VB-assisted UTB to bronchoscopists if deemed appropriate for the PNs (6).
The median diameter of the PNs in this study (2.6 cm) is similar, and it is slightly more peripheral (0.94 cm to pleura) than a previous meta-analysis (median diameter 2.5 cm, median distance to pleura 1.1 cm) (9). The diagnostic accuracy and sensitivity were 71.7% and 67.8% respectively in this study, similar to the pooled accuracy and sensitivity of 73.9% and 71.1% in previous meta-analysis (9). The sensitivity was lower than another meta-analysis with pooled sensitivity of 82% (10). However, it should be noted that our study included only patients who were highly unlikely to have the diagnosis made by other methods, short of a surgical biopsy. On the other hand, in the majority of previous studies, ENB was offered to patients who might simply have a lesion not accessible by conventional FB, without necessarily having tried other guiding methods (e.g., R-EBUS).
The NPV was low (30%), reflecting the high prevalence of serious pathologies in these PNs. The final diagnoses and outcomes of ENB-negative PNs were not well described in systematic review and meta-analyses of previous studies (6,9,10,14). In the first human study of 13 ENB cases, 4 ENB negative PNs were subsequently diagnosed to be malignant (17). In a number of individual studies, final diagnoses of ENB negative cases were not reported (11,18-20). In studies that have reported the final diagnoses of ENB negative PNs, the majority of lesions turned out to be malignant (7,15,16,21-26) with isolated reports of histoplasmosis and organising pneumonia (25). Our series confirm that malignancies are the commonest diagnoses in ENB-negative PNs, while TB is the next commonest problem, as expected in TB endemic regions, in contrast to previous studies in Western countries (Table 3). Benign tumours and organising pneumonia are occasional diagnoses we encounter. Results from ours and previous studies imply that these PNs need to be carefully followed up even if they are ENB negative, and often a surgical biopsy is required to make a final diagnosis.
Our study did not analyse the learning curves of the bronchoscopists. ENB is performed by 5 bronchoscopists in our centre, 2 are first generation ENB bronchoscopists with the longest experience, and 3 others are performing ENB under their supervision. This may have a negative impact on the diagnostic yield in this study. It was previously reported that the learning curve based on procedure time would become stable after 14 ENB procedures (27). On the other hand, experienced ENB bronchoscopists may select more difficult cases. So, the potential increase in diagnostic yield after gaining more experience maybe diluted by the selection of difficult cases. It would be interesting to see how the diagnostic yield evolves when a centre becomes more mature in performing this procedure over a few years.
Apart from training, cost (especially consumables) is currently a major barrier to a more widespread utilisation of ENB (14). The authors find it rewarding to make use of the VB features integral to the ENB system, and use UTB/R-EBUS/fluoroscopy to access the lesion in selected cases instead, to achieve some cost savings in terms of ENB consumables (13). In that sense the indication of ENB is still evolving lately in our centre, and we now only perform ENB in not accessible by UTB/VB/R-EBUS. They are probably the most difficult lesions to sample for a bronchoscopist.
In addition, institutions that have acquired the system can explore other areas of applications apart from diagnostic use, so that the ENB equipment can be fully utilised (13,28). For example, ENB has been successfully employed to dye mark small PNs to facilitate thoracoscopic surgical resection, especially when they are too small to be visualised or palpated during operation (29). Similarly, ENB allows accurate placement of fiducial markers in close proximity to malignant PNs to allow precise targeting in stereotactic body radiation therapy (SBRT) (30). ENB-guided placement of a conduit may also provide a good platform for emerging local treatment of malignant PNs, e.g., microwave, radiofrequency, radiation. These may allow patient to avoid surgery, and they potentially can offer diagnosis and treatment in the same session (28).
Conclusions
For PNs not accessible by conventional FB/R-EBUS and CT-TTNA, ENB provides good diagnostic yield with low complication rate. It is a useful armamentarium to respiratory physicians and thoracic surgeons. It may also become a useful platform for emerging therapeutic applications of malignant PNs, e.g., SBRT or local ablation.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Research Ethics Committee (Kowloon Central/Kowloon East Region), Hong Kong. Consent from the patient was waived. Standards for Reporting Diagnostic Accuracy Studies (STARD 2015) was used for reporting.
References
- Callister ME, Baldwin DR, Akram AR, et al. British Thoracic Society guidelines for the investigation and management of pulmonary nodules. Thorax 2015;70:ii1-54. [Crossref] [PubMed]
- Phua CK, Sim WY, Tee KS, et al. Evaluation of pulmonary nodules in Asian population. J Thorac Dis 2016;8:950-7. [Crossref] [PubMed]
- Bai C, Choi CM, Chu CM, et al. Evaluation of pulmonary nodules. Clinical practice consensus guidelines for Asia. Chest 2016;150:877-93. [Crossref] [PubMed]
- Rivera MP, Mehta AC; American College of Chest Physicians. Initial diagnosis of lung cancer. ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:131-48S.
- Wiener RS, Schwartz LM, Woloshin S, et al. Population-based risk for complications after transthoracic needle lung biopsy of a pulmonary nodule: an analysis of discharge records. Ann Intern Med 2011;155:137-44. [Crossref] [PubMed]
- Wang Memoli JS, Nietert PJ, Silvestri GA. Meta-analysis of guided bronchoscopy for the evaluation of the pulmonary nodule. Chest 2012;142:385-93. [Crossref] [PubMed]
- Eberhardt R, Anantham D, Ernst A, et al. Multimodality bronchoscopic diagnosis of peripheral lung lesions: a randomised controlled trial. Am J Respir Crit Care Med 2007;176:36-41. [Crossref] [PubMed]
- Leong S, Shaipanich T, Lam S, et al. Diagnostic bronchoscopy–current and future perspectives. J Thorac Dis 2013;5:S498-510. [PubMed]
- Gex G, Pralong JA, Combescure C, et al. Diagnostic yield and safety of electromagnetic navigation bronchoscopy for lung nodules: A systematic review and meta-analysis. Respiration 2014;87:165-76. [Crossref] [PubMed]
- Zhang W, Chen S, Dong X, et al. Meta-analysis of the diagnostic yield and safety of electromagnetic navigation bronchoscopy for lung nodules. J Thorac Dis 2015;7:799-809. [PubMed]
- Gu Y, Chen S, Shi J, et al. The introduction of electromagnetic navigation bronchoscopy for the diagnosis of small pulmonary peripheral lesions in an Asian population. J Thorac Dis 2017;9:2959-65. [Crossref] [PubMed]
- Du Rand IA, Blaikley J, Booton R, et al. British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults. Thorax 2013;68:i1-44. [Crossref] [PubMed]
- Cheng SL, Chu CM. Electromagnetic navigation bronchoscopy under intravenous sedation–tips and tricks. J Thorac Dis 2018;10:S769-74. [Crossref] [PubMed]
- Leong S, Ju H, Marshall H, et al. Electromagnetic navigation bronchoscopy: a descriptive analysis. J Thorac Dis 2012;4:173-85. [PubMed]
- Eberhardt R, Morgan RK, Ernst A, et al. Comparison of suction catheter versus forceps biopsy for sampling of solitary pulmonary nodules guided by electromagnetic navigation bronchoscopy. Respiration 2010;79:54-60. [Crossref] [PubMed]
- Seijo LM, de Torres JP, Lozano MD, et al. Diagnostic yield of electromagnetic navigation bronchoscopy is highly dependent on the presence of a bronchus sign on CT imaging. Results from a prospective study. Chest 2010;138:1316-21. [Crossref] [PubMed]
- Schwarz Y, Greif J, Becker HD, et al. Real-time electromagnetic navigation bronchoscopy to peripheral lung lesions using overlaid CT images. The first human study. Chest 2006;129:988-94. [Crossref] [PubMed]
- Becker HD, Herth F, Ernst A, et al. Bronchoscopic biopsy of peripheral lung lesions under electromagnetic guidance. A pilot study. J Bronchology Interv Pulmonol 2005;12:9-13.
- Wilson DS, Bartlett RJ. Improved diagnostic yield of bronchoscopy in a community practice: Combination of electromagnetic navigation system and rapid on-site evaluation. J Bronchol 2007;13:227-32. [Crossref]
- Lamprecht B, Porsch P, Wegleitner B, et al. Electromagnetic navigation bronchoscopy (ENB): Increasing diagnostic yield. Respir Med 2012;106:710-5. [Crossref] [PubMed]
- Gildea TR, Mazzone PJ, Karnak D, et al. Electromagnetic navigation diagnostic bronchoscopy. A prospective study. Am J Respir Crit Care Med 2006;174:982-9. [Crossref] [PubMed]
- Makris D, Scherpereel A, Leroy S, et al. Electromagnetic navigation diagnostic bronchoscopy for small peripheral lung lesions. Eur Respir J 2007;29:1187-92. [Crossref] [PubMed]
- Lamprecht B, Porsch P, Pirich C, et al. Electromagnetic navigation bronchoscopy in combination with PET-CT and rapid on-site cytopathologic examination for diagnosis of peripheral lung lesions. Lung 2009;187:55-9. [Crossref] [PubMed]
- Bertoletti L, Robert A, Cottier M, et al. Accuracy and feasibility of electromagnetic navigation bronchoscopy under nitrous oxide sedation for pulmonary peripheral opacities: an outpatient study. Respiration 2009;78:293-300. [Crossref] [PubMed]
- Mahajan AK, Patel S, Hogarth DK, et al. Electromagnetic navigation bronchoscopy. An effective and safe approach to diagnose peripheral lung lesions unreachable by conventional bronchoscopy in high-risk patients. J Bronchology Interv Pulmonol 2011;18:133-7. [Crossref] [PubMed]
- Pearlstein DP, Quinn CC, Burtis CC, et al. Electromagnetic navigation bronchoscopy performed by thoracic surgeons: one center’s early success. Ann Thorac Surg 2012;93:944-9; discussion 949-50. [Crossref] [PubMed]
- Sun J, Xie F, Zheng X, et al. Learning curve of electromagnetic navigation bronchoscopy for diagnosing peripheral pulmonary nodules in a single institution. Transl Cancer Res 2017;6:541-51. [Crossref]
- Port J, Harrison S. Electromagnetic navigational bronchoscopy. Semin Intervent Radiol 2013;30:128-32. [Crossref] [PubMed]
- Awais O, Reidy MR, Mehta K, et al. Electromagnetic navigation bronchoscopy-guided dye marking for thoracoscopic resection of pulmonary nodules. Ann Thorac Surg 2016;102:223-9. [Crossref] [PubMed]
- Nabavizadeh N, Zhang J, Elliot DA, et al. Electromagnetic navigation bronchoscopy-guided fiducial markers for lung stereotactic body radiation therapy: analysis of safety, feasibility and interfraction stability. J Bronchology Interv Pulmonol 2014;21:123-30. [Crossref] [PubMed]


