
Routine chest X-rays after the removal of chest tubes are not necessary following esophagectomy
Introduction
Esophageal cancer is the ninth most common cancer worldwide and remains an important cause of cancer death (1). Curative treatment is only possible for selected patients and consists of neoadjuvant therapy followed by esophagectomy (2). Since meticulous mediastinal lymphadenectomy is considered necessary to achieve an optimal oncological outcome, a transthoracic approach is preferred for esophagectomy by most surgeons (3-5).
To prevent potential postoperative pulmonary compression by accumulation of intrapleural air or fluid, placement of at least one chest tube is advised during esophagectomy (6). Chest tubes can generally be removed when producing less than 200 mL fluid per day and in case no air leak is observed (6). To diagnose potential hazardous situations, such as massive pneumothorax or re-accumulation of intrapleural fluid, it is common practice to perform a routine chest X-ray (CXR) several hours after removing a chest tube (7). However, studies that included patients who underwent a variety of cardiothoracic procedures (i.e., coronary artery bypass grafting, valve replacements, and other cardiothoracic procedures) indicated that radiological abnormalities upon routine CXRs after chest tube removal often do not require re-intervention and that clinical signs of respiratory problems might be appropriate criteria to guide further diagnostic imaging (8,9). In this light, the practice of routinely performing a CXR after chest tube removal has also become subject of debate for the postoperative care pathway of patients undergoing esophagectomy.
To date, no studies have evaluated the value of routinely performing a CXR after removing chest tubes during the postoperative course of esophagectomy. Therefore, the aim of this study was to investigate the clinical relevance of a routine CXR after chest tube removal in the postoperative management of esophagectomy.
Methods
Patients
A prospectively maintained database was used to select all patients who underwent esophagectomy with gastric conduit reconstruction in the University Medical Center Utrecht between 2015 and 2017. Patients who received a CXR within 4 hours after the removal of at least one chest tube according to available radiological reports were included. No specific exclusion criteria were defined. The current study was approved by the institutional review board and the need for written informed consent was waived.
Surgical procedure
Patients principally received a robot-assisted minimally invasive transthoracic esophagectomy with 2-field lymphadenectomy and gastric conduit reconstruction. A laparoscopic transhiatal approach was used for selected patients who were considered unfit for a transthoracic procedure, as discussed and agreed during multidisciplinary tumor board meetings. A hand-sewn esophagogastric anastomosis was created in all patients.
Management of chest tubes and drains
Both water seal and Jackson-Pratt (JP) chest tube were commonly placed during esophagectomy. The standard protocol included the placement of two or three chest tubes at the time of esophagectomy. At least one water seal chest tube was kept in place until postoperative day (POD) 1. From POD 1 onwards, removal of chest tubes was performed when the drained fluid volume was less than 200 cc/24 h, non-contaminated, and did not show signs of air leak. Chest tubes were pulled out at full expiration and a CXR was routinely performed 4 hours later to evaluate for accumulation of intrapleural air or fluid. All CXRs were assessed by radiologists who documented their findings in the electronical patient file. The necessity for re-intervention in terms of chest tube re-insertion was assessed by the attending surgeon based on the combination of radiological and clinical findings.
Data collection & outcome measures
Baseline characteristics were collected from a prospective database and consisted of age, gender, American Society of Anesthesiologists (ASA) score, tumor histology, clinical tumor stage, neoadjuvant therapy, and surgical approach. The dates of removing intraoperatively placed chest tubes were also prospectively recorded. The primary outcome measures included the proportion of patients with radiological signs of intrapleural air or fluid and the rate of chest tube re-insertion on the day of routine CXR or on the day afterwards. Radiological reports were retrieved from the electronic patient files and retrospectively evaluated for any quantity of intrapleural air or fluid on either side of the thorax. Clinical symptoms, respiratory rate, need for oxygen support, and oxygen saturation level at the time of routine CXR and at the time of deciding to re-insert a chest tube were also retrospectively collected from the electronic patient file. Patients who required chest tube re-insertion on the side of previous chest removal were identified by reviewing clinical documentation of the day and the day after chest tube removal in all patients.
Statistics
All analyses were performed using SPSS version 21 (SPSS Inc.). Continuous variables were expressed as a mean (standard deviation) or median (range or interquartile range), depending on data distribution. Categorical variables were presented as a number with the corresponding percentage. No statistical comparisons between groups were made.
Results
Patient characteristics
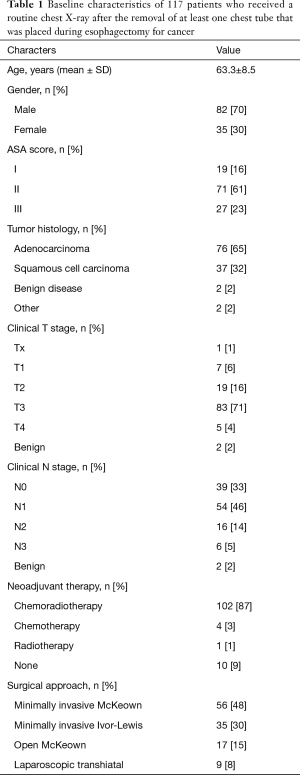
The baseline characteristics of the 117 patients who were included are demonstrated in Table 1. The majority of the patients was male (n=82, 70%) and the mean age was 64 years. Most patients presented with a tumor at clinical stage T3 or higher (n=88, 75%) and had at least one tumor-positive lymph node according to clinical staging (n=76, 65%). Neoadjuvant chemoradiotherapy was usually provided (n=102, 87%) and was followed by esophagectomy through a minimally invasive transthoracic (n=91, 78%), minimally invasive transhiatal (n=9, 8%), or open transthoracic (n=17, 15%) approach. The median day of removing the first chest tube was POD 3 (interquartile range, 1–4) and the median day of removing the last chest tube was POD 8 (range, 6–11).
Full table
Routine CXR findings
A CXR was performed after the removal of 231 intraoperatively placed chest tubes. Intrapleural air was seen on 78 CXRs (34%) and was a new finding on the ipsilateral side of previous chest tube removal in 33 cases (14%). Intrapleural fluid was mentioned in the radiological reports of 87 CXRs (38%), which involved a new finding that was ipsilateral to previous chest tube removal in 24 cases (10%).
Chest tube re-insertions
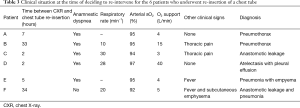
Out of the 231 chest tube removals that were followed by CXR, a chest tube was re-inserted in 6 cases (3%) and these re-interventions were performed 2 to 34 hours after the CXR. Table 2 demonstrates the clinical situation of these patients at the time of CXR, which had shown intrapleural air (n=3, 1%), intrapleural fluid (n=2, 1%), or none of these abnormalities (n=1, 1%). At the time of deciding to re-insert a chest tube, 3 of these patients had an increased respiratory rate (20, 28, and 30 breaths per minute), 1 patient had a decreased respiratory rate (10 breaths per minute), and in the remaining 2 patients no respiratory rate was documented. Oxygen support was required in all 6 of these patients (range, 3–40 L/min) to achieve acceptable saturation levels (range, 92–97%). All 6 patients who underwent chest tube re-insertion reported dyspnea at the time of CXR, at the time of deciding to re-insert a chest tube, or both.

Full table
Table 3 shows the other clinical signs and the ultimate diagnosis of the 6 patients who underwent chest tube re-insertion (patients A-F). Patients A and B were diagnosed with a pneumothorax in absence of other underlying pathology. Both had anamnestic dyspnea and a decreased respiratory rate was observed in patient B (10 breaths per minute), which may have been in response to thoracic pain that was present. The last CXR before chest tube removal had shown a small apical pneumothorax in both patients A and B. In patient C, who also received a new chest tube after showing intrapleural air upon the CXR after chest tube removal, anastomotic leakage was diagnosed by endoscopy on the same day. No signs of pneumothorax had been present on the last CXR prior to chest tube removal. In patients D and E, intrapleural fluid was observed on the CXR, which originated from atelectasis with pleural effusion (patient D) and pneumonia with empyema (patient E). The last CXR prior to chest tube removal had shown bilateral intrapleural fluid in both patients, which was most evident on the ipsilateral side of chest tube re-insertion. In patient F, no signs of intrapleural air or fluid were found upon CXR, but this patient developed fever and had subcutaneous emphysema due to a combination of anastomotic leakage and pneumonia (patient F). Although intrapleural air was not present on earlier CXR in the patient, subcutaneous emphysema was already present before removal of the chest tube.
Full table
Discussion
In this study, a CXR was routinely performed after chest tube removal, aiming to exclude clinically relevant intrapleural air or fluid as part of the standard postoperative protocol for esophagectomy. The chest tube re-insertion rate after the removal of chest tubes was 3%. The CXRs prior to this chest tube re-insertion showed intrapleural air in 1%, intrapleural fluid in another 1%, and none of these abnormalities in the remaining 1%. All patients who underwent chest tube re-insertion presented with clinical signs or symptoms.
The current findings suggest that intrapleural air and fluid are relatively common radiological findings after removing chest tubes following esophagectomy. Although the incidence of clinically insignificant pneumothorax is likely minimized by removing a chest tube at full expiration, a small proportion of patients is still expected to develop a clinically relevant pneumothorax after chest tube removal (10). In a study comparing chest tube removal at full expiration versus at full inspiration after pulmonary resection, a non-significant difference in clinically relevant pneumothoraces was found with an incidence of 1% in the former and 3% in the latter group (10). These rates appear to be in line with the current results and with another study that demonstrated that a CXR following chest tube removal rarely changes patient management (11,12).
In the current study, clinical signs or symptoms were present in all patients who underwent re-insertion of a chest tube. A pneumothorax without other pathology was ultimately diagnosed in one-third of these patients. The other two-thirds of these patients had clinical symptoms including fever, thoracic pain, and subcutaneous emphysema, which originated from another postoperative complication (i.e., anastomotic leakage, atelectasis, and pneumonia). All developed respiratory signs or symptoms that would have been reason to perform diagnostic tests, of which a CXR is often one of the first, mostly followed by computed tomography (CT) scanning. However, as 5 out of 6 patients who required re-insertion of a chest tube already showed signs of small amounts of intrapleural air or fluid on an earlier CXR, it should be emphasized that thorough evaluation of previous imaging is essential before removing any tubes. The decision to remove a drain under such clinical circumstances might be challenged, but it must be noted that non-productive chest tubes should principally be removed since they might otherwise only pose a risk of complications such as insertion site infection. To decide which patients need a CXR after chest tube removal, clinical appearance may be a suitable indicator. In a study that included patients who underwent various cardiothoracic surgical procedures (e.g., valve replacement, coronary artery bypass grafting), the cardiac surgeon scored the likelihood of finding a pneumothorax upon CXRs following chest tube removal, based on clinical characteristics. These characteristics included respiratory or hemodynamic changes (i.e., decreased oxygen saturation, dyspnea, tachypnea, decreased cardiac output, or significant drop in blood pressure) and problems that were experienced during chest tube removal (13). Although some small pneumothoraces occurred in patients who were classified as having the lowest level of suspicion, none of these patients required re-insertion of a chest tube (13).
Rational patient selection for postoperative imaging is in line with the increasing demands to effectively organize care and to optimally allocate expenses. The combination of available literature and current results suggests that a routine CXR after chest tube removal does not have to be part of the standard postoperative treatment pathway for esophagectomy, but may only be performed in patients who present with clinical signs or symptoms of respiratory problems. It should be noted, however, that the current study did not aim to identify clinical criteria that can be used to determine which patients need a CXR. Therefore, it seems advisable to clinically monitor patients after chest tube removal and to perform a CXR in case of any signs or symptoms that may be attributable to respiratory problems. Future research may strive to establish and validate a clinical scoring system to assess the need for a CXR in this context.
This study derives its strength from a largely prospective nature of data collection which resulted in reliable data. Furthermore, the aim of this paper was to provide insight in clinical decision making after removal of chest tubes, which was achieved by strictly assessing the value of the routine CXR. One might argue that using various types of tubes (i.e., water seal and JP tubes) might have interfered with the homogeneity of the cohort. However, this is not believed to be a hampering factor regarding the representability of results. Another limitation of the current study may be the retrospective nature of part of the data collection, which resulted in some missing parameters for a small group of patients.
In conclusion, this study suggests that the practice of routinely performing a CXR after chest tube removal following esophagectomy may safely be abandoned. Performing a CXR remains advisable in case of any signs or symptoms of respiratory problems after chest tube removal.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The current study was approved by the institutional review board and the need for written informed consent was waived.
References
- Lagergren J, Smyth E, Cunningham D, et al. Oesophageal cancer. Lancet 2017;390:2383-96. [Crossref] [PubMed]
- Shapiro J, van Lanschot JJB, Hulshof MCCM, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- Hulscher JB, van Sandick JW, de Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med 2002;347:1662-9. [Crossref] [PubMed]
- Omloo JM, Lagarde SM, Hulscher JB, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the mid/distal esophagus: five-year survival of a randomized clinical trial. Ann Surg 2007;246:992-1000; discussion 1000-1. [Crossref] [PubMed]
- Haverkamp L, Seesing MF, Ruurda JP, et al. Worldwide trends in surgical techniques in the treatment of esophageal and gastroesophageal junction cancer. Dis Esophagus 2017;30:1-7. [PubMed]
- Findlay JM, Gillies RS, Millo J, et al. Enhanced recovery for esophagectomy: a systematic review and evidence-based guidelines. Ann Surg 2014;259:413-31. [Crossref] [PubMed]
- Sepehripour AH, Farid S, Shah R. Is routine chest radiography indicated following chest drain removal after cardiothoracic surgery? Interact Cardiovasc Thorac Surg 2012;14:834-8. [Crossref] [PubMed]
- Khan T, Chawla G, Daniel R, et al. Is routine chest X-ray following mediastinal drain removal after cardiac surgery useful? Eur J Cardiothorac Surg 2008;34:542-4. [Crossref] [PubMed]
- Whitehouse MR, Patel A, Morgan JA. The necessity of routine post-thoracostomy tube chest radiographs in post-operative thoracic surgery patients. Surgeon 2009;7:79-81. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Skylizard L, et al. Optimal technique for the removal of chest tubes after pulmonary resection. J Thorac Cardiovasc Surg 2013;145:1535-9. [Crossref] [PubMed]
- Johnson B, Rylander M, Beres AL. Do X-rays after chest tube removal change patient management? J Pediatr Surg 2017;52:813-5. [Crossref] [PubMed]
- McCormick JT, O'Mara MS, Papasavas PK, et al. The use of routine chest X-ray films after chest tube removal in postoperative cardiac patients. Ann Thorac Surg 2002;74:2161-4. [Crossref] [PubMed]
- Eisenberg RL, Khabbaz KR. Are chest radiographs routinely indicated after chest tube removal following cardiac surgery? AJR Am J Roentgenol 2011;197:122-4. [Crossref] [PubMed]


