
Cardiac rhythm disorders in obstructive sleep apnea
Introduction
Obstructive sleep apnea (OSA) and cardiovascular diseases (CVD) commonly co-exist because of bi-directional mechanisms (1). Observational studies have shown that OSA is independently associated with CVD (1-3) and untreated OSA is associated with increased risk of cardiac events (4-6). In addition, OSA and CVD share several risk factors, including increasing age, sedentary life, and obesity, which help to explain the co-existence of both conditions in the same individuals. OSA is frequent in the general population, but it is strikingly common among patients with established hypertension (>30%) (7-9), heart failure (HF) (~40%) (10-12), and arrhythmias (~30%) (13-15). More than three decades ago, Guilleminault and colleagues reported a case series of 400 patients with OSA who underwent 24-hour Holter EKG. The authors showed that 48% had arrhythmias and conduction disturbances during sleep (16). Non-sustained ventricular tachycardia (VT), sinus arrest, and second-degree atrioventricular conduction block were the most significant rhythm disorders (16). Furthermore, among 50 patients who were treated with tracheostomy, the arrhythmias were resolved (16). Despite this compelling early evidence, OSA still remains largely underdiagnosed among patients with CVD (17). This may be partially explained by the perceived lack of studies clearly showing a causal relationship between OSA and CVD.
There is mounting evidence pointing that OSA may contribute to the development of cardiac rhythm disorders (13-17). For instance, treating OSA with continuous positive airway pressure (CPAP) reduced the frequency of ventricular premature beats during the sleep, in patients with heart failure (HF) (14). However, methodological limitations, such as the paucity of studies showing the clear mechanisms linking OSA and cardiac rhythm disorders, potential selection bias derived from observational studies, and lack of large randomized clinical trials are the main weakness of the field. Thus, the main goal of this article is to review the evidence that supports the hypothesis that OSA contributes to cardiac rhythm disorders development. To this end, we will first focus on the biological plausibility, by reviewing the potential mechanistic pathways linking OSA and cardiac arrhythmias. Among all arrhythmias, particular attention will be devoted to atrial fibrillation (AF). AF is the most prevalent arrhythmia worldwide (18) and is the arrhythmia most studied in association with OSA (19,20). In addition, attention will be devoted to the association between OSA and sudden cardiac death (SCD) (21). We have also combined several other arrhythmias potentially linked to OSA into a single topic. Finally, we will discuss future directions in the area. Due to the limited space, this review will not explore the link between central sleep apnea and arrhythmias, which frequently co-exist among patients with congestive HF (22,23).
Arrhythmogenesis
OSA is characterized by recurrent episodes of partial or complete upper airway occlusion during sleep (24). Several of the potential mechanisms linking OSA to arrhythmias overlap with the pathways relating OSA with other CVD (4-6). The primary potential mechanisms linking OSA and arrhythmias are: (I) excessive negative intrathoracic pressure swings that occur during futile efforts to breath against the occluded airway; (II) arousals from sleep at the end of obstructive events; (III) intermittent asphyxia characterized by hypoxia and hypercapnia. These three primary mechanisms occurring during sleep trigger a cascade of intermediate mechanisms that may augment acutely and chronically the propensity to arrhythmia (25-27). The two most studied pathways linking OSA and arrhythmias are intermittent hypoxia and sympathetic over activity (21,25-27). Increased sympathetic tone occurs during each episode of upper airway obstruction and sympathetic over activation remains during the wakefulness period in patients with OSA (21,28,29). Sympathetic discharges may trigger atrial activity abnormalities (30). In addition to acute mechanisms occurring during each obstructive event, OSA may lead to heart remodeling, that in turn, increases the propensity to cardiac arrhythmias. For the sake of clarity, we have divided the potential mechanisms linking OSA and arrhythmogenesis in subheads.
Heart remodeling
Atrial distension and remodeling are the anatomical substrates of atrial arrhythmias, in particular AF (31-33). In turn, OSA has been associated with atrial remodeling (34). For instance, OSA was associated with atrial conduction abnormalities related to connexin dysregulation and fibrosis in a rat model of repetitive obstructive events triggered by closing the airway at the end-expiration (35). Cardiac chambers distension and remodeling in patients with OSA may occur because of several mechanisms, including increased cardiac transmural pressure, surges in sympathetic activity and blood pressure that occur during each obstructive event (24). Cardiac over load may be also observed during the wakefulness due to sympathetic over activity that may contribute to high blood pressure (24). Atrial electromechanical alterations associated with increased risk of AF, including interatrial and intra-atrial electromechanical delay, as well as prolongation of P-wave dispersion have been observed in patients with moderate-to-severe OSA (34). Moreover, shortening of the atrial effective refractory period triggered by generation of negative intrathoracic pressure was observed in a pig model of OSA (36). Intrathoracic pressure shifts and surges in blood pressure lead to not only atrial consequences, but also ventricular hypertrophy and increased propensity to arrhythmias (37,38). Pathological left ventricular hypertrophy has been associated with sudden death (39).
Autonomic imbalance
The autonomic nervous system (ANS) modulates cardiac arrhythmogenesis (40-42). However, the role of ANS imbalance generating arrhythmias is still not fully understood, particularly because ANS modulation plays a different role according to specific arrhythmias (40). For instance, both sympathetic and parasympathetic activation may trigger AF. In contrast, sympathetic stimulation is pro-arrhythmic, whereas parasympathetic activation is anti-arrhythmic for ventricular fibrillation (VF) (40). In addition, an animal model showed that the stimulation of the right and the left vagus nerve has differential effects on atrioventricular conduction and heart rate (41). In this complex mechanistic scenario, the sympathovagal imbalance has been noted to be the key trigger in cardiac arrhythmogenesis in OSA patients (42). Increased sympathetic tone and decreased parasympathetic tone have been reported in patients with OSA (42,43). However, increased vagal tone has also been described in OSA patients (43). During each obstructive event, patients with OSA frequently have relative bradycardia due to surges in vagal tone, triggered by the diving reflex (43). Several studies have evaluated OSA patients using indirect measures of ANS activity, in particular heart rate variability (HRV) (42-45). A typical pattern of bradycardia-tachycardia represents the variation of cardiac ANS with bradycardia while apneic event and tachycardia at the end of apneic event (44,45). However, HRV should be interpreted with caution due to potential confounding effects of age, sex, and disease severity with HRV measures (46-48).
Oxidative stress and inflammation
OSA is a natural model of intermittent hypoxia, a well-known mechanism for the production of reactive oxygen species (ROS) (49). Oxidative stress was associated with autonomic dysfunction and hypertension in animal models (50). Similarly, in humans, OSA patients excessively generate ROS from leukocytes that have been ameliorated by the treatment of OSA patients with CPAP (51). However, there are some methodological limitations on these studies, such as observational design, small sample size, and inclusion of subjects with comorbidities (49-51). Thus, more research is needed to establish the effect of OSA treatment on oxidative stress. In the same context, intermittent hypoxia triggers inflammation (52). Inflammatory biomarkers have also been associated with OSA (53-55). Several inflammatory biomarkers such as C-reactive protein, interleukin-6, interleukin-8, tumor necrosis factor-alpha, and adhesion molecules have been associated with OSA (55). CPAP therapy has shown to decrease both inflammation and oxidative stress levels in OSA patients (56,57). On the other hand, there is increasing evidence that systemic inflammation plays a key role in AF development and also contributes to AF persistence (58). ROS also plays a role in producing arrhythmic substrate, especially in conditions such as diabetes and hypertension (59). Therefore, oxidative stress and inflammation contribute to increased arrhythmogenesis propensity (58,59) and they are mechanisms linked to OSA pathogenesis (49-57). These mechanistic pathways are potential therapeutic targets for reducing arrhythmias in OSA patients.
Atrial fibrillation (AF)
OSA has been associated with atrial perturbations that increase AF development susceptibility (Table 1) (25,26,34,36). In the Sleep Heart Health Study (SHHS) a multicenter, community-based with more than 6,000 participants, a cross sectional study showed that sleep-disordered breathing was independently associated with a four folder higher odds of AF compared to those without sleep-disordered breathing (60). Gami and colleagues studied consecutive patients undergoing electrical cardioversion for AF, who were compared to consecutive patients referred to a general cardiology practice, but without AF (61). They noted that the proportion of patients with OSA was higher in AF group compared to patients from a general cardiology practice (61). The odds ratio for the association between OSA and AF was 2.2 (61). Furthermore, another study showed that after a successful electrical cardioversion, untreated OSA was associated with a higher recurrence rate of AF than patients without OSA (62). AF recurrence was also more common after catheter ablation in patients with OSA (51%) than without OSA (30%) (63). Observational prospective studies are in line with these findings showing that OSA treatment with CPAP decreased the necessity of anti-arrhythmic drugs use as well as AF recurrence (64,65). Interestingly, the hazard ratio (HR) for AF recurrence after catheter ablation was higher for OSA severity (HR =2.61) than for left atrial volume measurement (HR =1.11), which is a well-recognized factor associated with AF development (65). In addition, the ORBIT-AF registry, with more than 10,000 participants, almost 2,000 subjects had OSA (13). AF patients with OSA had worse symptoms and higher risk of hospitalization than those without OSA (13).
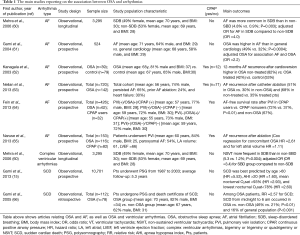
Full table
Most studies listed above (13,60-65), are observational in design, have small sample size, or registries that report on the association between OSA and AF. Thus, a recent published meta-analysis explored whether treating OSA with CPAP in patients with AF reduces recurrence rates of AF after catheter ablation (19). After exclusions, a total of eight studies were identified with total of 4,516 participants with AF that were submitted to catheter ablation. Of these, 1,247 subjects were diagnosed with OSA, but only 698 were treated with CPAP. Among those eight studies included, there was only one small randomized clinical trial with 83 subjects (67). The eight studies included were published between 2003 and 2013, the majority were men, age ranged from 50 to 66 years. The use of CPAP was associated with 44% decreased risk for AF recurrence (P<0.001) (19). The overall effect size was in favor to CPAP users in association of AF recurrence, showing a relative risk (RR) of 0.56 and 95% CI (0.47–0.68) (19). Therefore, there is evidence that patients with OSA treated with CPAP have lower AF recurrence rates after catheter ablation than patients with OSA without treatment (19). However, the evidence is based mainly on observational studies (19). Thus, randomized clinical trials are needed to establish the causal relationship of AF recurrence and OSA and strength clinical practice recommendations for treating OSA in patients with AF.
SCD and ventricular arrhythmias
SCD, resulting from ventricular arrhythmias, mainly VF, is still a significant clinical problem with highly annual rates in US population (68). As previously discussed, OSA-related to hypoxia, sympathovagal imbalance, and mechanical effects of negative intrathoracic pressure on the ventricular free walls are the key factors generating cardiac electrical abnormalities and increasing arrhythmias susceptibility (21,24,26,37,38,42). The SHHS showed that complex ventricular arrhythmias (defined as bigeminy or trigeminy or quadrigeminy or non-sustained VT) were more common in subjects with sleep-disordered breathing compared to those without sleep-disordered breathing, respectively (25.0% vs. 14.5%, respectively, P=0.002) (60). These complex ventricular arrhythmias may trigger spontaneous cardiac impulse formation and predispose to cardiac electrical repolarization changes that facilitate initiation of VF, the main arrhythmia associated with SCD (69). The main studies pointing to an association between OSA and complex ventricular arrhythmias and SCD are summarized in Table 1.
The event peak of SCD is from 6 a.m. to noon and has a nadir from midnight to 6 a.m. in the general population (66). In contrast to the pattern observed in the general population, Gami and colleagues showed that patients with OSA had a peak of SCD during the sleeping hours (66). The increased morning risk of SCD in the general population may be explained by factors that predispose to ischemia and arrhythmias, such as increased sympathetic activity (70), increased coagulability (71), and electrophysiological abnormalities (72). On the other hand, OSA has been associated with systemic inflammation (53-55,73), endothelial dysfunction (73,74), hypercoagulability (75-78), and oxidative stress (49-51). Thus, it is reasonable that OSA facilitates arrhythmogenesis and may lead to SCD during sleep. In line with the hypothesis that OSA predisposes to SCD, a longitudinal study of more than 10,000 participants, followed during an average of 5.3 years, showed that OSA, specifically nocturnal hypoxemia, was independently associated with SCD, even after adjusting for well-established risk factors such as age, hypertension, coronary artery disease, HF, and ventricular ectopy (15). Therefore, this longitudinal study showed the independent association between OSA and SCD, after adjusting for traditional cardiovascular risk factors, highlighting that OSA increases the propensity of cardiac arrhythmogenesis (15). Furthermore, there is also evidence for the association between OSA and ventricular arrhythmias including ventricular ectopy, which is potential trigger for VT or VF (79). However, there is no clear evidence for the correlation between OSA severity and increased risk for VT or VF (79). However, the majority of the studies reporting the association between ventricular arrhythmias and OSA are among patients with HF. HF per se is a well-established risk factor for increased arrhythmogenesis susceptibility (80). Having this potential bias in mind, several studies showed that both central sleep apnea and OSA were independently associated with higher frequency of ventricular arrhythmias (15,66,81-83). OSA was also associated with appropriate therapy by implantable cardioverter-defibrillator (ICD) device (81-83). Bitter and colleagues showed that the time to first ventricular arrhythmias occurrence and to first appropriate ICD therapy were significantly shorter in patients with OSA compared to those without or with mild OSA (81). Moreover, Serizawa and colleagues demonstrated that the presence of sleep-disordered breathing was common and an independent predictor of life-threatening ventricular arrhythmias in a HF population with ICD (82). The authors highlighted that these arrhythmias were more likely to occur during sleep (82). A higher incidence of appropriate ICD discharge was also shown in patients with sleep-disordered breathing and HF compared to those without sleep-disordered breathing and HF (83). However, some limitations merit consideration: only 17 patients were classified as having sleep-disordered breathing and of those 35% had central sleep apnea (83). Therefore, there is also a lack of studies with higher number of participants and longer follow-up period. Moreover, randomized studies evaluating the impact of OSA treatment are needed.
Other arrhythmias
Bradycardic rhythm disorders have been associated with OSA (84-86). Sleep apnea–induced hypoxemia is a key mechanism leading to an increased vagal tone and bradycardic rhythm disorders (84-86). For instance, Zwillich and colleagues showed that oxygen supplementation eliminated bradycardia (84). However, in the SHHS, sleep-disordered breathing was not significant associated with bradycardic rhythms and conduction delay arrhythmias (60). One potential explanation for the weaker association between conduction delay arrhythmias and sleep-disordered breathing was related to differences in ANS responses that could be mitigated in older individuals (60). A previous European multicenter study demonstrated a high prevalence of OSA (~60%) among patients with long-term pacing independent of the indication for pacing (87). However, paced patients with OSA were less symptomatic than typical patients with OSA and the significance of such high association remains to be established (87). Similarly, atrioventricular conduction block has been reported to be common in patients with OSA (16,85,86). However, the independent association between OSA and atrioventricular conduction block was also not shown in the SHHS (60). Some case reports showed an association between OSA and atrioventricular block (88-90) that were resolved after CPAP therapy (91). However, the impact of OSA treatment on bradycardic rhythms is still unclear and relies on studies with a limited sample size (88-91). For instance, one study evaluated 23 moderate-to-severe OSA patients with insertable loop recorder during 16 months and reported that almost 50% had severe nocturnal bradycardic events (91). CPAP treatment for 8 weeks was associated with a significant decrease in the median number of bradycardic episodes per patient (91).
The effects of sleep disordered breathing on increased arrhythmias susceptibility may be bi-directional. For instance, atrial overdrive stimulation may reduce both central sleep apnea and OSA (92). The rationale behind the mechanisms is not fully understood. One plausible explanation is that increasing heart rate and cardiac output by atrial overdrive stimulation, would lead to reduction in circulation time, decrease in pulmonary congestion, and neck fluid accumulation (92,93). In turn, these mechanisms may contribute to the pathogenesis of central sleep apnea and OSA. A meta-analysis evaluating the effects of atrial overdrive pacing included a total of eight randomized trials with only 129 patients (92). It is important to point out that the participants had HF with predominance of central sleep apnea in 3 out of 8 studies. The pooled analyzes showed that atrial overdrive pacing reduced sleep apnea in patients with central sleep apnea-predominantly. However, no significant effects were observed in patients with OSA (92). Finally, OSA has also been common among patients with typical atrial flutter (94). However, in contrast to AF as previously described, OSA was not a predictor of arrhythmia recurrence after catheter ablation in patients with atrial flutter (94). This observation reinforces the need to clear elucidate the specificity of the association between OSA and each rhythm disorder.
Future directions
There is consistent evidence that nocturnal arrhythmias are common in patients with OSA. There are several biological pathways that may explain the link between OSA and increased cardiac arrhythmogenesis propensity. Intermittent hypoxia, intrathoracic pressure shifts, acting synergistically with increased sympathetic activation and blood pressure surges, play a key role in electromechanical cardiac abnormalities, increasing arrhythmias development susceptibility. However, the evidence of the beneficial effects of OSA treatment on arrhythmias is derived from observational studies and small sized clinical trials. Therefore, large randomized clinical trials are needed to point out future directions for treatment with evidence-based guidelines. In the precision medicine area, a better understanding of different OSA phenotypes and specific pathways, which contribute to cardiac electrical remodeling, may be important for targeting future therapy.
Acknowledgements
The authors thank the Lemann Foundation for supporting education in the research area. Dr. Geovanini was supported by the Lemann Foundation during her postdoctoral fellowship at Brigham and Women’s Hospital-Harvard Medical School, Boston-MA, USA (from July 2015 to July 2017).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation 2010;122:352-60. [Crossref] [PubMed]
- Javaheri S, Blackwell T, Ancoli-Israel S, et al. Sleep-disordered Breathing and Incident Heart Failure in Older Men. Am J Respir Crit Care Med 2016;193:561-8. [Crossref] [PubMed]
- Roca GQ, Redline S, Claggett B, et al. Sex-specific association of sleep apnea severity with subclinical myocardial injury, ventricular hypertrophy, and heart failure risk in a community-dwelling cohort: The Atherosclerosis Risk in Communities–Sleep Heart Health Study. Circulation 2015;132:1329-37. [Crossref] [PubMed]
- Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005;365:1046-53. [Crossref] [PubMed]
- Campos-Rodriguez F, Martinez-Garcia MA. Cardiovascular mortality in women with obstructive sleep apnea with or without continuous positive airway pressure treatment: a cohort study. Ann Intern Med 2012;156:115-22. [Crossref] [PubMed]
- Barbé F, Durán-Cantolla J, Sánchez-de-la-Torre M, et al. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA 2012;307:2161-8. [Crossref] [PubMed]
- Haentjens P, Van Meerhaeghe A, Moscariello A, et al. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trials. Arch Intern Med 2007;167:757-64. [Crossref] [PubMed]
- Montesi SB, Edwards BA, Malhotra A, et al. The effect of continuous positive airway pressure treatment on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J Clin Sleep Med 2012;8:587-96. [PubMed]
- Pedrosa RP, Drager LF, de Paula LKG, et al. Effects of OSA treatment on BP in patients with resistant hypertension: a randomized trial. Chest 2013;144:1487-94. [Crossref] [PubMed]
- Herrscher TE, Akre H, Øverland B, et al. High prevalence of sleep apnea in heart failure outpatients: even in patients with preserved systolic function. J Card Fail 2011;17:420-5. [Crossref] [PubMed]
- Bitter T, Faber L, Hering D, et al. Sleep-disordered breathing in heart failure with normal left ventricular ejection fraction. Eur J Heart Fail 2009;11:602-8. [Crossref] [PubMed]
- Oldenburg O, Wellmann B, Buchholz A, et al. Nocturnal hypoxaemia is associated with increased mortality in stable heart failure patients. Eur Heart J 2016;37:1695-703. [Crossref] [PubMed]
- Holmqvist F, Guan N, Zhu Z, et al. Impact of obstructive sleep apnea and continuous positive airway pressure therapy on outcomes in patients with atrial fibrillation-results from the outcomes registry for better informed treatment of atrial fibrillation (ORBIT-AF). Am Heart J 2015;169:647-54.e2. [Crossref] [PubMed]
- Ryan CM, Usui K, Floras JS, et al. Effect of continuous positive airway pressure on ventricular ectopy in heart failure patients with obstructive sleep apnoea. Thorax 2005;60:781-5. [Crossref] [PubMed]
- Gami AS, Olson EJ, Shen WK, et al. Obstructive sleep apnea and the risk of sudden cardiac death: a longitudinal study of 10,701 adults. J Am Coll Cardiol 2013;62:610-6. [Crossref] [PubMed]
- Guilleminault C, Connolly SJ, Winkle RA. Cardiac arrhythmia and conduction disturbances during sleep in 400 patients with sleep apnea syndrome. Am J Cardiol 1983;52:490-4. [Crossref] [PubMed]
- Costa LE, Uchôa CH, Harmon RR, et al. Potential underdiagnosis of obstructive sleep apnoea in the cardiology outpatient setting. Heart 2015;101:1288-92. [Crossref] [PubMed]
- Benjamin EJ, Chen PS, Bild DE, et al. Prevention of atrial fibrillation: report from a national heart, lung, and blood institute workshop. Circulation 2009;119:606-18. [Crossref] [PubMed]
- Qureshi WT, Nasir UB, Alqalyoobi S, et al. Meta-analysis of continuous positive airway pressure as a therapy of atrial fibrillation in obstructive sleep apnea. Am J Cardiol 2015;116:1767-73. [Crossref] [PubMed]
- Genta PR, Drager LF, Lorenzi Filho G. Screening for Obstructive Sleep Apnea in Patients with Atrial Fibrillation. Sleep Med Clin 2017;12:99-105. [Crossref] [PubMed]
- Zipes DP, Rubart M. Neural modulation of cardiac arrhythmias and sudden cardiac death. Heart Rhythm 2006;3:108-13. [Crossref] [PubMed]
- Abraham JM, Connolly SJ. Atrial fibrillation in heart failure: stroke risk stratification and anticoagulation. Heart Fail Rev 2014;19:305-13. [Crossref] [PubMed]
- Thihalolipavan S, Morin DP. Atrial fibrillation and heart failure: update 2015. Prog Cardiovasc Dis 2015;58:126-35. [Crossref] [PubMed]
- Dempsey JA, Veasey SC, Morgan BJ, et al. Pathophysiology of sleep apnea. Physiol Rev 2010;90:47-112. [Crossref] [PubMed]
- May AM, Van Wagoner DR, Mehra R. OSA and Cardiac Arrhythmogenesis: Mechanistic Insights. Chest 2017;151:225-41. [Crossref] [PubMed]
- Baranchuk A. Sleep apnea, cardiac arrhythmias, and conduction disorders. J Electrocardiol 2012;45:508-12. [Crossref] [PubMed]
- Patel N, Donahue C, Shenoy A, et al. Obstructive sleep apnea and arrhythmia: A systemic review. Int J Cardiol 2017;228:967-70. [Crossref] [PubMed]
- Khayat R, Patt B, Hayes D. Obstructive sleep apnea: the new cardiovascular disease. Part I: obstructive sleep apnea and the pathogenesis of vascular disease. Heart Fail Rev 2009;14:143-53. [Crossref] [PubMed]
- Souvannakitti D, Kumar GK, Fox A, et al. Contrasting effects of intermittent and continuous hypoxia on low O(2) evoked catecholamine secretion from neonatal rat chromaffin cells. Adv Exp Med Biol 2009;648:345-9. [Crossref] [PubMed]
- Volders PGA. Novel insights into the role of the sympathetic nervous system in cardiac arrhythmogenesis. Heart Rhythm 2010;7:1900-6. [Crossref] [PubMed]
- Baranchuk A, Parfrey B, Lim L, et al. Interatrial block in patients with obstructive sleep apnea. Cardiol J 2011;18:171. [PubMed]
- Kammersgaard LP, Olsen TS. Cardiovascular risk factors and 5-year mortality in the Copenhagen Stroke Study. Cerebrovasc Dis 2006;21:187-93. [Crossref] [PubMed]
- Benjamin EJ, Wolf PA, D'Agostino RB, et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 1998;98:946-52. [Crossref] [PubMed]
- Yagmur J, Yetkin O, Cansel M, et al. Assessment of atrial electromechanical delay and influential factors in patients with obstructive sleep apnea. Sleep Breath 2012;16:83-8. [Crossref] [PubMed]
- Iwasaki YK, Kato T, Xiong F, et al. Atrial fibrillation promotion with long-term repetitive obstructive sleep apnea in a rat model. J Am Coll Cardiol 2014;64:2013-23. [Crossref] [PubMed]
- Linz D, Schotten U, Neuberger H-R, et al. Combined blockade of early and late activated atrial potassium currents suppresses atrial fibrillation in a pig model of obstructive apnea. Heart Rhythm 2011;8:1933-9. [Crossref] [PubMed]
- Cioffi G, Russo TE, Stefenelli C, et al. Severe obstructive sleep apnea elicits concentric left ventricular geometry. J Hypertens 2010;28:1074-82. [Crossref] [PubMed]
- Orban M, Bruce CJ, Pressman GS, et al. Dynamic Changes of Left Ventricular Performance and Left Atrial Volume Induced by the Mueller Maneuver in Healthy Young Adults and Implications for Obstructive Sleep Apnea, Atrial Fibrillation, and Heart Failure. Am J Cardiol 2008;102:1557-61. [Crossref] [PubMed]
- Chelliah RK, Senior R. Pathological and physiological left ventricular hypertrophy: echocardiography for differentiation. Future Cardiol 2009;5:495-502. [Crossref] [PubMed]
- Shen MJ, Zipes DP. Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ Res 2014;114:1004-21. [Crossref] [PubMed]
- Ng GA, Brack KE, Coote JH. Effects of direct sympathetic and vagus nerve stimulation on the physiology of the whole heart–a novel model of isolated Langendorff perfused rabbit heart with intact dual autonomic innervation. Exp Physiol 2001;86:319-29. [Crossref] [PubMed]
- Jo JA, Blasi A, Valladares E, et al. Determinants of heart rate variability in obstructive sleep apnea syndrome during wakefulness and sleep. Am J Physiol Heart Circ Physiol 2005;288:H1103. [Crossref] [PubMed]
- Leung RS. Sleep-disordered breathing: autonomic mechanisms and arrhythmias. Prog Cardiovasc Dis 2009;51:324-38. [Crossref] [PubMed]
- Guilleminault C, Connolly S, Windle R, et al. Cyclic variation of the heart rate in sleep apnea syndrome. Mechanisms, and usefulness of 24 h electrocardiography as a screening technique. Lancet 1984;1:126-31. [Crossref] [PubMed]
- Hayano J, Watanabe E, Saito Y, et al. Screening for obstructive sleep apnea by cyclic variation of heart rate. Circ Arrhythm Electrophysiol 2011;4:64-72. [Crossref] [PubMed]
- Kuo TB, Lin T, Yang CC, et al. Effect of aging on gender differences in neural control of heart rate. Am J Physiol 1999;277:H2233-9. [PubMed]
- Narkiewicz K, Montano N, Cogliati C, et al. Altered cardiovascular variability in obstructive sleep apnea. Circulation 1998;98:1071-7. [Crossref] [PubMed]
- Wang W, Tretriluxana S, Redline S, et al. Association of cardiac autonomic function measures with severity of sleep-disordered breathing in a community-based sample. J Sleep Res 2008;17:251-62. [Crossref] [PubMed]
- Nanduri J, Vaddi DR, Khan SA, et al. Xanthine oxidase mediates hypoxia-inducible factor-2α degradation by intermittent hypoxia. PLoS One 2013;8:e75838. [Crossref] [PubMed]
- Nanduri J, Wang N, Yuan G, et al. Intermittent hypoxia degrades HIF-2alpha via calpains resulting in oxidative stress: implications for recurrent apnea-induced morbidities. Proc Natl Acad Sci U S A 2009;106:1199-204. [Crossref] [PubMed]
- Eisele HJ, Markart P, Schulz R. Obstructive Sleep Apnea, Oxidative Stress, and Cardiovascular Disease: Evidence from Human Studies. Oxid Med Cell Longev 2015;2015:608438. [Crossref] [PubMed]
- May AM, Mehra R. Obstructive sleep apnea: role of intermittent hypoxia and inflammation. Semin Respir Crit Care Med 2014;35:531-44. [Crossref] [PubMed]
- Unnikrishnan D, Jun J, Polotsky V. Inflammation in sleep apnea: an update. Rev Endocr Metab Disord 2015;16:25-34. [Crossref] [PubMed]
- Geovanini GR, Wang R, Weng J, et al. Elevations in neutrophils with obstructive sleep apnea: The Multi-Ethnic Study of Atherosclerosis (MESA). Int J Cardiol 2018;257:318-23. [Crossref] [PubMed]
- Nadeem R, Molnar J, Madbouly EM, et al. Serum inflammatory markers in obstructive sleep apnea: a meta-analysis. J Clin Sleep Med 2013;9:1003-12. [PubMed]
- Karamanlı H, Özol D, Ugur KS, et al. Influence of CPAP treatment on airway and systemic inflammation in OSAS patients. Sleep Breath 2014;18:251-6. [Crossref] [PubMed]
- Murri M, García-Delgado R, Alcázar-Ramírez J, et al. Effect of CPAP on oxidative stress and circulating progenitor cell levels in sleep patients with apnea-hypopnea syndrome. Respir Care 2011;56:1830-6. [Crossref] [PubMed]
- Chung MK, Martin DO, Sprecher D, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation 2001;104:2886-91. [Crossref] [PubMed]
- Tse G, Yan BP, Chan YW, et al. Reactive Oxygen Species, Endoplasmic Reticulum Stress and Mitochondrial Dysfunction: The Link with Cardiac Arrhythmogenesis. Front Physiol 2016;7:313. [Crossref] [PubMed]
- Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am J Respir Crit Care Med 2006;173:910-6. [Crossref] [PubMed]
- Gami AS, Pressman G, Caples SM, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation 2004;110:364e367.
- Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation 2003;107:2589-94. [Crossref] [PubMed]
- Neilan TG, Farhad H, Dodson JA, et al. Effect of sleep apnea and continuous positive airway pressure on cardiac structure and recurrence of atrial fibrillation. J Am Heart Assoc 2013;2:e000421. [Crossref] [PubMed]
- Fein AS, Shvilkin A, Shah D, et al. Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation recurrence after catheter ablation. J Am Coll Cardiol 2013;62:300-5. [Crossref] [PubMed]
- Naruse Y, Tada H, Satoh M, et al. Concomitant obstructive sleep apnea increases the recurrence of atrial fibrillation following radiofrequency catheter ablation of atrial fibrillation: clinical impact of continuous positive airway pressure therapy. Heart Rhythm 2013;10:331-7. [Crossref] [PubMed]
- Gami AS, Howard DE, Olson EJ, et al. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med 2005;352:1206-14. [Crossref] [PubMed]
- Craig S, Pepperell JC, Kohler M, et al. Continuous positive airway pressure treatment for obstructive sleep apnoea reduces resting heart rate but does not affect dysrhythmias: a randomised controlled trial. J Sleep Res 2009;18:329-36. [Crossref] [PubMed]
- Writing Group Members. Heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation 2010;121:e46-215. [PubMed]
- Yanowitz F, Preston JB, Abildskov JA. Functional distribution of right and left stellate innervation to the ventricles. Production of neurogenic electrocardiographic changes by unilateral alteration of sympathetic tone. Circ Res 1966;18:416-28. [Crossref] [PubMed]
- Somers VK, Dyken ME, Mark AL, et al. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med 1993;328:303-7. [Crossref] [PubMed]
- Andreotti F, Davies GJ, Hackett DR, et al. Major circadian fluctuations in fibrinolytic factors and possible relevance to time of onset of myocardial infarction, sudden cardiac death and stroke. Am J Cardiol 1988;62:635-7. [Crossref] [PubMed]
- Huikuri HH, Yli-Mayry S, Linnaluoto MK, et al. Diurnal fluctuations in human ventricular and atrial refractoriness. Pacing Clin Electrophysiol 1995;18:1362-8. [Crossref] [PubMed]
- Zychowski KE, Sanchez B, Pedrosa RP, et al. Serum from obstructive sleep apnea patients induces inflammatory responses in coronary artery endothelial cells. Atherosclerosis 2016;254:59-66. [Crossref] [PubMed]
- Drager LF, Polotsky VY, O'Donnell CP, et al. Translational approaches to understanding metabolic dysfunction and cardiovascular consequences of obstructive sleep apnea. Am J Physiol Heart Circ Physiol 2015;309:H1101-11. [Crossref] [PubMed]
- Liak C, Fitzpatrick M. Coagulability in obstructive sleep apnea. Can Respir J 2011;18:338-48. [Crossref] [PubMed]
- Rahangdale S, Yeh SY, Novack V, et al. The influence of intermittent hypoxemia on platelet activation in obese patients with obstructive sleep apnea. J Clin Sleep Med 2011;7:172-8. [PubMed]
- Nakanishi K, Tajima F, Nakata Y, et al. Hypercoagulable state in a hypobaric, hypoxic environment causes non-bacterial thrombotic endocarditis in rats. J Pathol 1997;181:338-46. [Crossref] [PubMed]
- Wessendorf TE, Thilmann AF, Wang YM, et al. Fibrinogen levels and obstructive sleep apnea in ischemic stroke. Am J Respir Crit Care Med 2000;162:2039-42. [Crossref] [PubMed]
- Raghuram A, Clay R, Kumbam A, et al. A Systematic Review of the Association between Obstructive Sleep Apnea and Ventricular Arrhythmias. J Clin Sleep Med 2014;10:1155-60. [PubMed]
- Saltzman HE. Arrhythmias and heart failure. Cardiol Clin 2014;32:125-33. [Crossref] [PubMed]
- Bitter T, Westerheide N, Prinz C, et al. Cheyne-Stokes respiration and obstructive sleep apnoea are independent risk factors for malignant ventricular arrhythmias requiring appropriate cardioverter-defibrillator therapies in patients with congestive heart failure. Eur Heart J 2011;32:61-74. [Crossref] [PubMed]
- Serizawa N, Yumino D, Kajimoto K, et al. Impact of sleep-disordered breathing on life-threatening ventricular arrhythmia in heart failure patients with implantable cardioverter-defibrillator. Am J Cardiol 2008;102:1064-8. [Crossref] [PubMed]
- Tomaello L, Zanolla L, Vassanelli C, et al. Sleep disordered breathing is associated with appropriate implantable cardioverter defibrillator therapy in congestive heart failure patients. Clin Cardiol 2010;33:E27-30. [Crossref] [PubMed]
- Zwillich C, Devlin T, White D, et al. Bradycardia during sleep apnea: characteristics and mechanism. J Clin Invest 1982;69:1286-92. [Crossref] [PubMed]
- Becker HF, Koehler U, Stammnitz A, et al. Heart block in patients with sleep apnoea. Thorax 1998;53:S29-32. [Crossref] [PubMed]
- Grimm W, Hoffmann J, Menz V, et al. Electrophysiologic evaluation of sinus node function and atrioventricular conduction in patients with prolonged ventricular asystole during obstructive sleep apnea. Am J Cardiol 1996;77:1310-4. [Crossref] [PubMed]
- Garrigue S, Pépin JL, Defaye P, et al. High prevalence of sleep apnea syndrome in patients with long-term pacing: the European Multicenter Polysomnographic Study. Circulation 2007;115:1703-9. [Crossref] [PubMed]
- Kawana F, Kasai T, Maeno K, et al. Atrioventricular block during the phasic events of REM sleep in a patient with severe obstructive sleep apnea syndrome. J Clin Sleep Med 2008;4:257-9. [PubMed]
- Maeno K, Kasai A, Setsuda M, et al. Advanced atrioventricular block induced by obstructive sleep apnea before oxygen desaturation. Heart Vessels 2009;24:236-40. [Crossref] [PubMed]
- Ji KH, Kim DH, Yun CH. Severe obstructive sleep apnea syndrome with symptomatic daytime bradyarrhythmia. J Clin Sleep Med 2009;5:246-7. [PubMed]
- Simantirakis EN, Schiza SI, Marketou ME, et al. Severe bradyarrhythmias in patients with sleep apnoea: the effect of continuous positive airway pressure treatment: a long-term evaluation using an insertable loop recorder. Eur Heart J 2004;25:1070-6. [Crossref] [PubMed]
- Weng CL, Chen Q, Ma YL, et al. A meta-analysis of the effects of atrial overdrive pacing on sleep apnea syndrome. Pacing Clin Electrophysiol 2009;32:1434-43. [Crossref] [PubMed]
- White LH, Motwani S, Kasai T, et al. Effect of rostral fluid shift on pharyngeal resistance in men with and without obstructive sleep apnea. Respir Physiol Neurobiol 2014;192:17-22. [Crossref] [PubMed]
- van Oosten EM, Furqan MA, Redfearn DP, et al. Sleep apnea does not predict atrial flutter recurrence after atrial flutter ablation. J Interv Card Electrophysiol 2012;34:73-8. [Crossref] [PubMed]


