Spread through air spaces in lung cancer patients is a risk factor for pulmonary metastasis after surgery
Introduction
While outcomes of lung cancer surgery are improving (1), recurrence develops in approximately 20–30% of patients, even after complete resection is performed (2-5). Among patients who undergo anatomical lung resection for early-stage non-small cell lung cancer (NSCLC), distant metastasis is the most common type of recurrence (2). The lung is the most common metastatic site (3). Although lymphovascular invasion has been reported to be a risk factor for distant recurrence (3), few studies have reported these recurrence patterns in detail. Moreover, the mechanisms of metastasis have not been well clarified.
In 2015, the concept of spread through air spaces (STAS), which is an indicator of lung cancer invasiveness, was defined in the World Health Organization (WHO) guidelines (6,7). These guidelines state that “STAS consists of micropapillary clusters, solid nests, or single cells beyond the edge of the tumor into air spaces in the surrounding lung parenchyma” (6). Despite the criticism suggesting that STAS is a type of artifact (8) and STAS is still under investigation, many reports have confirmed that STAS is a true, invasive lung cancer pattern and is a significant predictive factor for both recurrence and survival (9-19). However, lung cancer recurrence patterns have not been clarified in patients with STAS. Kadota et al. (10) and our group (20) previously showed that patients with STAS who underwent sublobar resection experienced a significantly higher incidence of locoregional recurrence and pulmonary metastasis compared to other patients, but we could not clearly demonstrate that STAS is associated with pulmonary metastasis.
As lung cancer with STAS is suspected to spread easily via air spaces, it is assumed to have the capability to metastasize throughout the lung. The aim of this study was to explore STAS and recurrence patterns in patients with lung cancer, and to elucidate the contribution of STAS to pulmonary metastasis after lung cancer surgery.
Methods
Study design
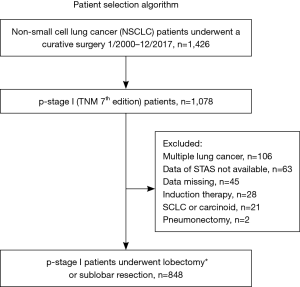
This was a retrospective study based on our prospectively maintained institutional database (13,20) which includes the data of all patients who underwent thoracic surgery in our institute. Since we established the database in May 2004, data prior to May 2004 were retrospectively abstracted from medical records and pathologic slides. Between January 2000 and December 2017, a total of 1,426 patients underwent complete resection of NSCLC. Staging was based on both clinical and pathological TNM stage (International Union Against Cancer staging system, 7th edition) (21). Our database included the following data: (I) patient demographics [age, sex, smoking status, tumor markers including serum carcinoembryonic antigen (CEA) level, comorbidities, and pulmonary function tests]; (II) information regarding the diagnosis [diagnostic method and radiological findings of thin-section chest computed tomography (CT) and positron emission tomography (PET)/CT]; (III) surgical procedures; (IV) pathological findings (grading, size, lymph node metastasis, lymphovascular invasion, pleural invasion, and STAS); and (V) outcomes (site of recurrence, death, and follow-up). Among 1,426 patients, those who had multiple lung cancers, had data of STAS not available, received induction chemotherapy, had small cell carcinoma or carcinoid tumors, who underwent pneumonectomy, and had data missing were all excluded. After excluding these patients, we studied 848 patients with pathological stage I disease who underwent lobectomy or sublobar resection (Figure 1). Lobectomy with mediastinal lymph node dissection was performed as a standard technique for NSCLC. Patients with a poor performance status, impaired respiratory function, and/or severe comorbidities underwent sublobar resection.
Pathological examination
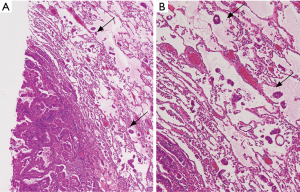
Surgically resected specimens were fixed in 10% formalin, sectioned into slices of 5–10 mm thickness, and stained with hematoxylin and eosin (HE). The presence of pleural, lymphatic, and vascular invasion was routinely examined. Verhoeff’s elastic stain was used to evaluate pleural and vascular invasion. Evaluation of STAS has been described in our previous reports (13,20). Namely, STAS was defined as the presence of tumor clusters lying freely within the alveolar space, at a distance of at least 0.5 mm from the primary tumor (13,20) (Figure 2). When only a few clusters were present in alveolar spaces, they were considered to be artificial floating tumor clusters, not STAS. The authors (Satoshi Shiono and Naoki Yanagawa) discussed the pathological findings of each case to achieve consensus.
Postoperative surveillance
Follow-up surveillance consisted of a visit 1 or 2 weeks after surgery and every month thereafter for up to 3 months, except for patients who received adjuvant therapy. For patients who did not appear to experience recurrence within the first 3 months, follow-up was performed every 3–4 months for 5 years. Regular outpatient clinic check-ups, including physical examination, blood chemistry, serum tumor markers, and chest radiography, were performed 2–4 times each year for 5 years. During the follow-up period, patients underwent annual chest and brain CT imaging. After 5 years, chest radiography or CT was performed annually. In patients with abnormal imaging or tumor marker findings yet for whom recurrence was difficult to diagnose, frequent surveillance CT or PET/CT was performed.
Initial recurrence sites were recorded in the database; only initial recurrences were evaluated. We defined locoregional recurrences as surgical margin recurrences, hilar and mediastinal lymph node metastases, ipsilateral pulmonary metastases, and pleural dissemination. Distant recurrences included extrathoracic organ and contralateral pulmonary metastases. Pulmonary metastasis was defined as a new or enlarging pulmonary nodule on follow-up CT or a hyper-metabolic nodule on follow-up PET/CT. Pulmonary metastasis included ipsilateral and contralateral metastasis. Biopsy was not mandatory to confirm pathological diagnosis of recurrence.
For validity and consistency, all recurrences were judged by a multidisciplinary team including a thoracic surgeon, a pulmonologist, a radiologist, and a pathologist. Recurrence-free rates and recurrence patterns were determined by both clinical factors and pathological findings, with particular attention paid to the presence of STAS.
Statistical analysis
The chi-squared test was used to evaluate the associations between categorical variables and various factors, and the Wilcoxon rank sum test was used to evaluate continuous variables. The length of follow-up was defined as the time from the day of surgery to the day of last hospital visit or death from any cause. Overall survival and recurrence-free rates were estimated using the Kaplan-Meier method, and evaluated by log-rank tests. Overall survival was measured from the date of lung cancer surgery to either the date of death from any cause or the last hospital visit. Recurrence-free rate was measured from the date of surgery to the date of detection of recurrence. When possible, the date of pathological diagnosis was defined as the date of recurrence. Patients who died from other causes were censored. Univariate and multivariate Cox proportional hazards regression analyses were used to identify prognostic factors for recurrence. For multivariate analysis, significant factors in univariate analysis (P<0.05) were selected. Data were analyzed using JMP software, version 5.0.1J (SAS Institute Inc., Cary, NC, USA). A P value less than 0.05 was considered to indicate statistical significance.
Ethical approval
The Ethics Committee of our institution approved this study (Ethics Committee approval No. 99) and, since the patient data remained anonymous, waived the need for informed consent from the patients.
Results
Clinicopathological characteristics recurrence and survival of patients
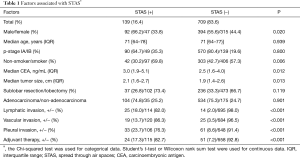
During the study period, 660 patients with pathological stage IA NSCLC and 188 patients with stage IB NSCLC were evaluated. Median follow-up was 60 months using the Kaplan-Meier estimate. With respect to surgical procedures, 570 (67.2%) patients underwent lobectomy, 6 (0.7%) underwent bilobectomy, 152 (17.9%) underwent segmentectomy, and 121 (14.3%) underwent wedge resection. With respect to histology, 638 (75.2%) patients had adenocarcinoma, 176 (20.8%) had squamous cell carcinoma, 11 (1.3%) had adenosquamous cell carcinoma, 10 (1.2%) had large cell neuroendocrine carcinoma, 8 (0.9%) had large cell carcinoma, and 5 (0.6%) had pleomorphic carcinoma. A total of 39 (4.6%) patients had lymphatic invasion, 44 (5.2%) had vascular invasion, 94 (11.1%) had pleural invasion, and 139 (16.4%) had STAS. Patient with STAS had more invasive characteristics compared to patients without STAS (Table 1). No deaths occurred within 30 days of surgery. A total of 152 deaths occurred, including 57 cancer-related deaths. The overall 5-year survival rate among patients with stage IA disease was 84.2%, and that among patients with stage IB disease was 76.1% (P=0.014). The 5-year recurrence-free survival rate among patients with stage IA disease was 89.5% and that among patients with stage IB disease was 70.6% (P<0.001).
Full table
Recurrence
Recurrence occurred in 108 (12.7%) patients. Locations of recurrence included the lungs (n=46), hilar lymph nodes (n=17), mediastinal lymph nodes (n=15), pleura (n=12), brain (n=10), bone (n=9), liver (n=4), adrenal gland (n=3), supraclavicular lymph node (n=1), and skin (n=1). Recurrences were classified as locoregional recurrences in 76 patients (9.0%), distant metastases in 27 patients (3.2%), and both in 5 patients (0.6%). Recurrence developed in 68 of 575 (11.8%) lobectomy cases, 12 of 152 (7.9%) segmentectomy cases, and 28 of 121 (23.1%) wedge resection cases; the recurrence rate among patients who underwent wedge resection was significantly higher than in those who underwent segmentectomy (P<0.001).
Risk factors for recurrence
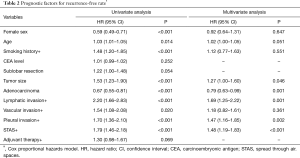
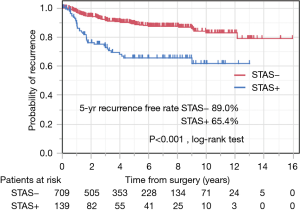
Table 2 lists the risk factors for recurrence-free survival. Multivariate analysis showed that large tumor size, non-adenocarcinoma histology, lymphatic invasion, pleural invasion, and STAS positivity were significant risk factors for recurrence (Table 2). The recurrence-free rate of the patients who were positive for STAS was significantly worse than in the negative patients. Five-year recurrence-free rates were 65.4% among patients with STAS and 89.0% among patients without STAS (P<0.001) (Figure 3).
Full table
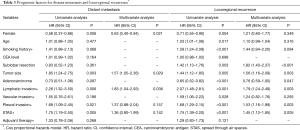
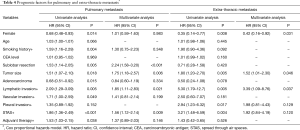
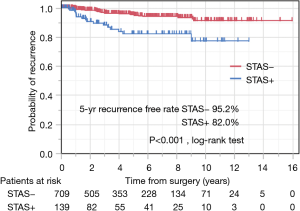
Risk factors for recurrence were evaluated by type of recurrence. For distant metastasis, multivariate analysis showed that male sex, large tumor size, and lymphatic invasion were significant risk factors for distant metastasis (Table 3). Additionally, multivariate analysis revealed that sublobar resection, large tumor size, non-adenocarcinoma histology, lymphatic invasion, pleural invasion, and STAS were significant risk factors for locoregional recurrence (Table 3). For pulmonary metastasis, multivariate analysis showed that sublobar resection, large tumor size, lymphatic invasion, and STAS were significant risk factors (Table 4). The recurrence-free rate of pulmonary metastasis of the patients who were positive for STAS was significantly worse than in the negative patients. Five-year recurrence-free rates of pulmonary metastasis of patients with STAS and without STAS were 95.2% and 82.0%, respectively (P<0.001) (Figure 4). Finally, male sex, large tumor size, and lymphatic invasion were identified as significant risk factors for extrathoracic recurrence in multivariate analysis (Table 4).
Full table
Full table
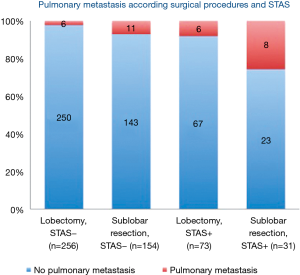
When a wide margin is achieved in patients with lung cancer with STAS, it is assumed that lobectomy can prevent pulmonary metastasis. Patients with STAS who underwent sublobar resection had a significantly higher rate of pulmonary metastasis (24.3%) compared to patients with STAS who underwent lobectomy (0.8%) (P=0.014) (Figure 5). This suggests that lobectomy might be able to prevent pulmonary metastasis, even in cases with STAS.
Discussion
Patients with stage I NSCLC may experience disease recurrence (3-5). In a study of 1,294 patients with completely resected stage I–II lung cancers, 67 (5.2%) had locoregional recurrence, 112 (8.7%) had distant metastasis, and 78 (6.0%) experienced both types of recurrence (22). The most common site of recurrence is the lung. Taylor et al. observed 141 (37.3%) cases of pulmonary metastasis among 378 recurrences (3). Yoshino et al. reported that pulmonary metastasis developed in 45 of 118 (38.1%) lung cancer patients with lung cancer recurrence, followed by bone metastasis in 35 of 118 (30.0%) patients and brain metastasis in 30 of 118 (25.4%) patients (23). However, since the mechanisms underlying pulmonary metastasis are not well clarified, we hypothesized that STAS could metastasize to another part of the lung, and explored whether STAS was a cause of pulmonary metastasis. The present results indicate that STAS can indeed contribute to pulmonary metastasis.
The association between STAS and recurrence patterns has also not been clarified. The results of our recent study suggested that STAS might be a risk factor for pulmonary metastasis (20), and we have updated our data and studied the relationships between STAS and pulmonary metastasis using multivariate analysis. Interestingly, although STAS is a risk factor for locoregional and pulmonary metastasis, it is not a risk factor for extrathoracic metastasis. Thus, STAS is only associated with intrapulmonary metastasis, including that from surgical margins. However, how floating cancer cell clusters implant in the alveolar space remains unclear. Further study of cell adhesion molecules is required to elucidate this phenomenon.
We found that patients with STAS who underwent sublobar resection had higher recurrence rates compared to patients who underwent other types of surgical resection. STAS is speculated to be a substantial risk factor not only for locoregional recurrence but also for pulmonary metastasis among patients who undergo sublobar resection.
We considered that lobectomy should be sufficient for prevention of pulmonary metastasis among patients with STAS. Mollberg and Ferguson noted that patients who undergo parenchyma-sparing surgery, i.e., sublobar resection, might lose any benefit of “collateral treatment” from removal of tissue at risk for recurrence (24). With respect to pulmonary metastasis, our data support the benefit of “collateral treatment.” Therefore, lobectomy appears to be a reasonable method for reducing recurrence, consistent with results of previous studies (25,26).
Regarding recurrence, other pathological factors observed in patients with stage I NSCLC have been reported (27,28). Our group showed that visceral pleural invasion was associated with local recurrence (27). Koike et al. revealed that wedge resection, microscopic positive margins, visceral pleural invasion, and lymphatic invasion were predictors of locoregional recurrence (28). However, in their study, 34 (10.4%) patients developed pulmonary metastasis in the same lobe, and no risk factors for pulmonary metastasis were identified.
If STAS can be predicted prior to surgery, particularly in sublobar resection cases, locoregional recurrence or pulmonary metastasis could be potentially avoided. Solid nodules on CT have been identified as significant indicators of STAS by multivariate analysis (13). In another report, a higher consolidation/tumor ratio and higher maximum standard uptake value on PET/CT were associated with STAS (19). Unfortunately, in the present study, we could not assess the CT findings of patients prior to 2004. However, chest CT findings might provide useful information for prediction of STAS.
Adjuvant chemotherapy has been proposed for the prevention of recurrence (29). In the present study, adjuvant therapy was more frequently administered to patients with STAS after surgery. However, adjuvant therapy did not appear to be more effective in STAS-positive cases (P=0.989). Moreover, the present results did not show a positive impact of adjuvant therapy among all patients. We speculate the reason for this is because only patients with stage I lung cancer were enrolled in the present study.
This study has some limitations. The main limitation is that we could not confirm recurrences using invasive testing. Depending on the location, the diagnosis of recurrence was not possible. In a study by Lou et al., 5% of patients underwent invasive testing to diagnose recurrence, and no deaths resulted from complications of invasive testing (22). However, since invasive testing may be associated with severe complications, we elected to use non-invasive testing, e.g., frequent surveillance CT or PET/CT, to evaluate recurrence in the present study. We also differentiated pulmonary metastasis from second primary lung cancer using radiological findings, clinical course, and pathological findings based on Martini-Melamed criterion (30). The possibility of second primary lung cancer cannot completely be excluded. The second limitation of this study is that some patients were investigated retrospectively, although we prospectively collected patient data. More prospective data collection is required to confirm the present results.
In conclusion, among pathological stage I lung cancer patients, STAS was frequently observed in patients with invasive disease. In patients who underwent complete resection of lung cancer, patients with STAS tended to develop locoregional recurrence and pulmonary metastases. Sublobar resection should be indicated, with caution, for patients suspected to have STAS.
Acknowledgements
None.
Footnote
Conflicts of Interest: Presented at the 26th European Conference on General Thoracic Surgery, Ljubljana, Slovenia, May 27–30, 2018.
Ethical Statement: The Ethics Committee of our institution approved this study (Ethics Committee approval No. 99) and, since the patient data remained anonymous, waived the need for informed consent from the patients.
References
- Sawabata N, Miyaoka E, Asamura H, et al. Japanese lung cancer registry study of 11,663 surgical cases in 2004: demographic and prognosis changes over decade. J Thorac Oncol 2011;6:1229-35. [Crossref] [PubMed]
- Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non–small cell carcinoma: Results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011;141:662-70. [Crossref] [PubMed]
- Taylor MD, Nagji AS, Bhamidipati CM, et al. Tumor recurrence after complete resection for non-small cell lung cancer. Ann Thorac Surg 2012;93:1813-20; discussion 1820-1.
- Demicheli R, Fornili M, Ambogi F, et al. Recurrence dynamics for non-small cell lung cancer. Effect of surgery on the development of metastases. J Thorac Oncol 2012;7:723-30. [Crossref] [PubMed]
- Murthy SC, Reznik SI, Ogwudu UC, et al. Winning the battle, losing the war: the noncurative ‘‘curative’’ resection for stage I adenocarcinoma of the lung. Ann Thorac Surg 2010;90:1067-74. [Crossref] [PubMed]
- Travis WD, Brambilla E, Burke AP, et al. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. Lyon: International Agency for Research on Cancer, 2015.
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World health organization classification of lung tumors: Impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Blaauwgeers H, Flieder D, Warth A, et al. A prospective study of loose tissue fragments in non–small cell lung cancer resection specimens. An Alternative View to “Spread Through Air Spaces”. Am J Surg Pathol 2017;41:1226-30. [Crossref] [PubMed]
- Onozato ML, Kovach AE, Yeap BY, et al. Tumor islands in resected early stage lung adenocarcinomas are associated with unique clinicopathological and molecular characteristics and worse prognosis. Am J Surg Pathol 2013;37:287-94. [Crossref] [PubMed]
- Kadota K, Nitadori J, Sima CS, et al. Tumor spread through air spaces is an important pattern of invasion and impacts the frequency and location of recurrences after limited resection for small stage I lung adenocarcinomas. J Thorac Oncol 2015;10:806-14. [Crossref] [PubMed]
- Warth A, Muley T, Kossakowski CA, et al. Prognostic impact of intra-alveolar tumor spread in pulmonary adenocarcinomas. Am J Surg Pathol 2015;39:793-801. [Crossref] [PubMed]
- Morimoto J, Nakajima T, Suzuki H, et al. Impact of free tumor clusters on prognosis after resection of pulmonary adenocarcinoma. J Thorac Cardiovasc Surg 2016;152:64-72.e1. [Crossref] [PubMed]
- Shiono S, Yanagawa N. Spread through air spaces is a predictive factor of recurrence and prognostic factor in stage I lung adenocarcinoma. Interact Cardiovasc Thorac Surg 2016;23:567-72. [Crossref] [PubMed]
- Lu S, Tan KS, Kadota K, et al. Spread through air spaces (STAS) is an independent predictor of recurrence and lung cancer–specific death in squamous cell carcinoma. J Thorac Oncol 2017;12:223-34. [Crossref] [PubMed]
- Uruga H, Fujii T, Fujimori S, et al. Semiquantitative assessment of tumor spread through air spaces (STAS) in early-stage lung adenocarcinomas. J Thorac Oncol 2017;12:1046-51. [Crossref] [PubMed]
- Dai C, Xie H, Su H, et al. Tumor spread thorough air spaces affects the recurrence and overall survival in patients with lung adenocarcinoma >2 to 3cm. J Thorac Oncol 2017;12:1052-60. [Crossref] [PubMed]
- Kadota K, Kushida Y, Katuski N, et al. Tumor spread through air spaces is an independent predictor of recurrence-free survival in patients with resected lung squamous cell carcinoma. Am J Surg Pathol 2017;41:1077-86. [Crossref] [PubMed]
- Masai K, Sakurai H, Sukeda A, et al. Prognostic impact of margin distance and tumor spread through air spaces in limited resection for primary lung cancer. J Thorac Oncol 2017;12:1788-97. [Crossref] [PubMed]
- Toyokawa G, Yamada Y, Tagawa T, et al. Significance of spread through air spaces in resected pathological stage I lung adenocarcinoma. Ann Thorac Surg 2018;105:1655-63. [Crossref] [PubMed]
- Shiono S, Endo M, Suzuki K, et al. Spread through air spaces Is a prognostic factor in sublobar resection of non-small cell lung cancer. Ann Thorac Surg 2018;106:354-360. [Crossref] [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. International Association for the Study of Lung Cancer International Staging Committee. Participating Institutions. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [Crossref] [PubMed]
- Lou F, Huang J, Sima CS, et al. Patterns of recurrence and second primary lung cancer in early-stage lung cancer survivors followed with routine computed tomography surveillance. J Thorac Cardiovasc Surg 2013;145:75-81; discussion 81-2. [Crossref] [PubMed]
- Yoshino I, Yohena T, Kitajima M, et al. Survival of non-small cell lung cancer patients with postoperative recurrence at distant organs. Ann Thorac Cardiovasc Surg 2001;7:204-9. [PubMed]
- Mollberg NM, Ferguson MK. Postoperative surveillance for non-small cell lung cancer resected with curative intent: Developing a patient-centered approach. Ann Thorac Surg 2013;95:1112-21. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22. [Crossref] [PubMed]
- Khullar OV, Liu Y, Gillespie T, et al. Survival after sublobar resection versus lobectomy for clinical stage IA lung cancer. J Thorac Oncol 2015;10:1625-33. [Crossref] [PubMed]
- Yanagawa N, Shiono S, Abiko M, et al. Tamura G. Prognostic impact and initial recurrence site of lymphovascular and visceral pleural invasion in surgically resected stage I non-small-cell lung carcinoma. Eur J Cardiothorac Surg 2013;44:e200-6. [Crossref] [PubMed]
- Koike T, Koike T, Yoshiya K, et al. Risk factor analysis of locoregional recurrence after sublobar resection in patients with clinical stage IA non-small cell lung cancer. J Thorac Cardiovasc Surg 2013;146:372-8. [Crossref] [PubMed]
- Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE collaborative group. J Clin Oncol 2008;26:3552-9. [Crossref] [PubMed]
- Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg 1975;70:606-12. [PubMed]