
Changes in quality of life after surgery or radiotherapy in early-stage lung cancer
Introduction
Surgical resection has been considered the standard of care and most effective treatment for early-stage non-small cell lung cancer (NSCLC) (1,2). However, approximately 25% of early-stage NSCLC patients do not undergo surgery due preexisting comorbidities, older age, or refusal (1,3). stereotactic body radiation therapy (SBRT), also known as stereotactic ablative radiotherapy (SABR), has been proposed in order to provide a minimally invasive treatment that improves accuracy in delivering ultra-high radiation doses (4). SBRT has become accepted and adapted as an alternative treatment for early-stage NSCLC (5-11).
To date, there is no consensus on the comparative effectiveness of SBRT versus surgery (12-14). Two randomized controlled trials [STARS (15) and ROSEL (16)] attempted to compare SBRT and surgery in the treatment of NSCLC, however, both trials were closed early due to low recruitment. A pooled analysis of these two trials suggested a better 3-year survival with SBRT in comparison to surgery (5). However, a meta-analysis comparing the effectiveness of SBRT and surgical resection in early-stage NSCLC found that 3-year survival of sublobar resection (SLR) and SBRT were comparable (6). Several retrospective studies have reported comparable outcomes between surgery and SBRT (7-10).
Early-stage NSCLC patients receiving SBRT may differ from patients eligible for surgery: they tend to be older, have more comorbidities and lower baseline health-related quality of life (HRQoL) than surgical patients (9,16,17). The toxicity associated with SBRT and its appropriateness for elderly patients or less healthy patients is still a topic of debate (7,8,17-20). Similarly, there remains a lack of consensus surrounding HRQoL after surgery for early-stage NSCLC. Several studies have found a decrease in post-operative HRQoL compared with pre-operative levels (21-23). However, others report that deficits in HRQoL increase in the long-term beyond baseline levels (22). While an abundance of literature focuses on the comparative effectiveness of surgery versus SBRT (7-10,13-16) and the impacts of each individually on HRQoL (20-27), no studies, to our knowledge, have examined the differences in quality of life (QoL) between SBRT and surgery in early-stage NSCLC patients. The high incidence and improved survival for NSCLC necessitate a close examination of potential differential QoL between surgery and SBRT in early-stage NSCLC patients, as many early-stage survivors are able to live long lives post-treatment. Coupled with the unique challenges faced by lung cancer survivors including physical (22,28-32) and mental health difficulties (32-36), differential QoL between the two treatment options could have important implications for patient and provider decision-making (37).
We hypothesize that SBRT will confer less of a negative impact on physical HRQoL from pre- to post-treatment as compared to surgery overall given the more invasive nature of surgery. However, our previous work has indicated that early-stage NSCLC SLR patients show very little HRQoL changes as compared to slight decrease from pre- to post-surgery in physical HRQoL among lobectomy patients (21,31). Therefore, we hypothesize that surgical patients and SBRT patients will both demonstrate decreases in physical HRQoL, however, within surgical patients, lobectomy patients will demonstrate worse physical HRQoL decreases as compared to SLR patients. Further, we expect that SLR patients will demonstrate similar decreases in HRQoL to SBRT patients. Based on previous research, we do not expect to see significant changes in mental HRQoL in either the surgical or SBRT patients.
Methods
Data source and patient population
This study used the Surveillance, Epidemiology, and End Results Medicare Health Outcomes Survey (SEER-MHOS) data set. Starting in 1998, the Center for Medicare and Medicaid Services has annually surveyed approximately 1,000 to 1,200 randomly selected beneficiaries from each participating managed care organization in the Medicare Advantage program, in order to gather clinically meaningful data on health outcomes, including functional status, comorbid conditions, and HRQoL. Selected beneficiaries are administered a baseline survey, and a follow-up survey 2 years later (38). This data was later linked to SEER, allowing for assessment of HRQoL in relation to cancer diagnoses and treatments. The Icahn Medical School at Mount Sinai Review Board for Health Sciences Research considered this study exempt because it relies on existing data without patient identifiers.
SEER-MHOS was queried from 1998 to 2014 for all patients with a first primary diagnosis of lung cancer, with surveys before and after treatment for first cancer diagnosis. Because some beneficiaries may have been selected for more than one MHOS cohort, in some cases the first survey was the follow-up from the earlier cohort, while the second survey was the baseline from a later cohort. For our purposes, baseline survey refers to the most recent survey prior to diagnosis and treatment and the follow-up survey refers to first survey after treatment. Those with a gap between surveys longer than ~2.5 years were excluded. Analysis was limited to patients with a microscopically confirmed diagnosis of a stage 0 or I lung cancer, who underwent only surgery or SBRT (n=184) (See supplementary appendix online for Figure S1 which contains the complete selection criteria).
Outcomes
HRQoL was measured using the 36-item Short Form Health Survey (SF-36) until 2006, when it was replaced by the Veterans RAND 12-Item Health Survey (VR-12) instrument. Physical Component Summary (PCS) and Mental Component Summary (MCS) scores were calculated based on individual subscale scores (including physical functioning, physical role limitation, pain, general health, emotional well-being, emotional role limitation, social functioning, and energy). The PCS and MCS scores have been normalized to the 1990 US general population (mean ± standard deviation, 50±10) and rescored to be equivalent across all cohorts/years of data collection.
Statistical analyses
Continuous variables (PCS and MCS) are reported as means and standard deviations, while categorical variables (all covariates) are reported as percentages. Differences between the two treatment groups at baseline were compared using χ2 tests (or Fisher’s exact test, where appropriate) for categorical variables and t-tests for continuous variables. Paired t-tests were used to assess changes over time in PCS and MCS scores from baseline to follow-up within each treatment group. Univariate and multivariate repeated measures analysis of variance (ANOVA) were performed to compare the change over time in the SBRT and surgery groups, by evaluating the interaction between treatment and time. This analysis was repeated to compare the sub-group of patients who underwent either lobectomy or SLR. Additionally, the analysis was repeated among those who underwent SLR or SBRT, since these two treatments are sometimes seen as alternatives for patients with poorer baseline health. All analyses were adjusted for age at diagnosis, gender, race, highest level of education attained, smoking status, and whether each survey was completed by proxy. When there was a significant difference in the presence of a comorbidity between the groups, the analysis was adjusted for the presence of that comorbidity. Comorbidities were assessed using the MHOS survey questions, and included hypertension (HTN), angina pectoris/coronary artery disease (CAD), congestive heart failure (CHF), myocardial infarction, stroke, emphysema/asthma/chronic obstructive pulmonary disease (COPD), and diabetes. For covariates with missing or unknown values, a “missing” category was created so as not to exclude those patients in multivariable analyses. Surgical and SBRT groups were also compared using a 1:1 propensity score matching with the Greedy algorithm on age at diagnosis, gender, race, education, baseline smoking status, completion of survey by proxy, and presence of emphysema/asthma/COPD, and angina pectoris/CAD. All statistical analyses were conducted using SAS software (version 9.4, SAS Institute Inc., Cary, NC). All P values are derived from two-tailed tests.
Results
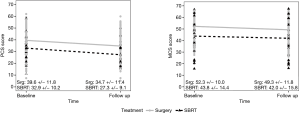
There were 184 patients (28 with SBRT, 156 with surgery) who matched the selection criteria. The average time between surveys was 2.0 years (range, 0.8–2.3 years). The baseline surveys were completed, on average, 1 year before diagnosis and treatment (range, 0.02–2.12 years) and the follow-up surveys 1 year after diagnosis (range, 0.04–2.12 years). This did not significantly differ between the two groups (i.e., surgery and SBRT). Patients from both groups experienced a significant decline in PCS score, of −4.81 from the baseline to follow-up in surgery patients (95% CI: −6.31,−3.30; P<0.0001), while SBRT patients experienced a change of −5.6 (95% CI: −9.96, −1.24; P=0.0137). For MCS scores, surgery patients experienced a change of −2.96 (95% CI: −4.55, −1.37; P=0.0003), while SBRT patients experienced a non-significant change of −1.86 (95% CI: −5.4, 1.68; P=0.2902) (Figure 1). There were significant differences in baseline PCS (P=0.0061) and MCS (P=0.0056) values between patients who underwent surgery and those who underwent SBRT, with those in the surgery group having higher baseline values for both. Patients in the SBRT group were older, with lower levels of education, though not significantly. Patients treated with SBRT were significantly more likely to have reported COPD, emphysema, or asthma (P<0.0001), and angina pectoris/CAD (P=0.0108).
Both the univariate and multivariate analyses revealed no significant difference in the change over time between the two treatment options for either PCS or MCS score (Table 1). A propensity matched analysis resulted in 22 patients in each group. There were no significant differences in QoL changes between the two groups, however, the baseline differences in HRQoL were not significant between surgery and SBRT, and the change in PCS score from baseline to follow-up among surgical patients was no longer significant. (Tables S1,S2 and Figure S2 in the supplemental appendix online).

Full table

Full table

Full table
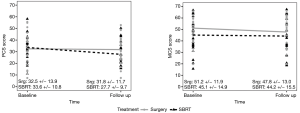
Among surgery patients, there were 128 who received a lobectomy and 26 a SLR. Lobectomy patients experienced a significant change in PCS score (−4.62, 95% CI: −6.3, −2.93; P<0.0001) from before to after surgery, as did SLR patients (−5.15, 95% CI: −8.84, −1.46; P=0.0081). Lobectomy patients also experienced a significant change in MCS score (−3.11, 95% CI: −4.74, −1.48; P=0.0002), while the results for SLR patients were not significant (−3.12, 95% CI: −8.01, 1.80; P=0.2035) (Figure 2). There was no significant difference between PCS and MCS scores at baseline between the two surgery types. Patients receiving a lobectomy were more likely to have a higher level of education (P=0.0294) and less likely to have their baseline and follow-up survey filled out by a proxy (P=0.0037 and P=0.0011 respectively). Lobectomy patients were also significantly less likely to be smokers at the time of the follow-up survey (P=0.0032).
Both the univariate and multivariate analyses showed no significant difference in the change over time between the two types of surgery for either PCS or MCS score (Table 1).
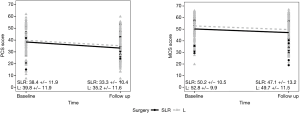
Patients who underwent either SBRT (n=28) or SLR (n=26) were compared directly. Both groups of patients experienced a significant decline in PCS score from baseline to follow-up, while the change for MCS scores were not significant (Figure 3). Though not statistically significant, there were some differences between SLR and SBRT patients in baseline PCS score (P=0.0744) and MCS score (P=0.0700). SLR patients were significantly more likely to be smokers at their baseline survey (P=0.0293), though that difference was not present at follow-up. SBRT patients were significantly more likely to have reported COPD, emphysema, or asthma (P=0.0039), and angina pectoris or CAD (P=0.0250); they were also more likely to have diabetes (P=0.0607).

No significant differences in the change over time, as measured by the treatment*time interaction were found in the univariate and multivariate models for PCS score or MCS score (Table 1).
Discussion
Confirming our hypothesis, patients from both the surgical and SBRT groups demonstrated decreases in physical HRQoL, but, inconsistent with our hypothesis, the magnitude of the decline did not vary between groups. The only significant differences between the two groups was in baseline physical and mental HRQoL, with SBRT patients having worse QoL in both areas. This could be due to the fact that the SBRT patients were likely sicker given their significantly higher likelihood of having lung-related comorbidities and heart disease and the fact that they were slightly older. The decreases in mental HRQoL, although significant for surgical patients, were quite modest and do not represent a meaningful decline in emotional wellbeing. This is consistent with our previous work involving surgical patients only, in which mental HRQoL was relatively consistent across time points (24,34). It is possible that, despite the decline in physical HRQoL, there is a feeling of relief post-treatment that may counteract the potential negative impact of physical discomfort associated with treatment. It would be important to measure mental HRQoL at later time points though, given that recent qualitative research points to experiences of anxiety, depression and isolation that can persist long after the treatment has been completed (37).
Based on our previous work and the current findings that suggested that lobectomy conferred a greater negative impact on physical HRQoL as compared to SLR (34), we compared SBRT and SLR directly, expecting that SLR and SBRT patients would demonstrate a similar decline in physical HRQoL as compared to SBRT patients. Support for this hypothesis was found as there were no significant differences between the two groups. Physical HRQoL decreased in both groups although the rate of these decreases was not different between the two groups. Similar to the overall surgery group, the baseline physical and mental HRQoL scores were slightly lower for the SBRT group than for the SLR group, again likely due to increased prevalence of comorbidities.
Given the small sample sizes, the results of the study should be considered preliminary, however, the results suggest that surgery (regardless of type) and SBRT are relatively comparable in terms of the impact on physical and mental HRQoL and suggest that SBRT is a good alternative for those for whom surgery is contraindicated. The similar impact on HRQoL was somewhat surprising given the less invasive nature of SBRT, however it is likely that the selection bias in terms of who receives SBRT (i.e., older, sicker patients) may account for a greater likelihood of experiencing slight negative physical HRQoL impacts. Also, it is important to note that a certain percentage of patients die during surgical treatment unlike SBRT in which death is highly unlikely; this may have biased the sample, since data from the sickest, most at-risk surgical patients who die during surgery would not have been included in the study.
Study results imply that treating physicians, whether they are surgeons or radiotherapists, need to consider the impact of treatment on HRQoL. It is important to discuss and prepare patients for these impacts so that social support and post-treatment care is in place ahead of surgery. Results from our qualitative study also suggested that coordination with a nurse navigator or someone in a similar type of role would be greatly beneficial to helping reduce the negative HRQoL impacts on early-stage lung cancer patients (37).
Strengths of this analysis are the access to the SEER-MHOS data set, which allowed for the examination of cases from a large, representative patient data pool, and the possibility to directly compare QoL changes after surgery and radiotherapy. A limitation, however, is that after applying the inclusion criteria, particularly the need for HRQoL measurement at both a pre- and post-treatment time point, the sample size was ultimately quite reduced. An analysis of differences at baseline between those who completed a follow-up survey and those who did not revealed that the latter were significantly more likely to be treated with SBRT. Similar differences were observed with the group who was not included due to death after the first survey. Additionally, those who were not included because they did not have a valid baseline or follow-up were significantly older and also more likely to receive SBRT than those who were included. These all point to the possibility that the oldest and sickest patients were not included in the current study and that they were more likely to have been treated with SBRT. Perhaps the HRQoL scores would have appeared even worse for the SBRT group had these patients been included. Additionally, although patients completed the two surveys 1 year prior and 1 year after diagnosis on average, the variability in the time frame could impact the results. It is possible that someone completed a HRQoL follow-up survey immediately following surgery, and that may result in a worse HRQoL as compared to someone who had completed the survey a year or more after treatment. Similarly, the longer time between diagnosis and baseline survey completion, the more likely the patient was in better health. These factors could create HRQoL changes that vary more as a function of time since treatment than just time between baseline and follow-up. However, upon analysis, the time between surveys did not significantly vary by treatment group (i.e., surgery and SBRT) thereby limiting the impact that any survey time frame variability could have on HRQoL differences between treatment groups.
As more patients are diagnosed with early-stage lung cancers due to increased screening, it is likely that large databases such as such as SEER-MHOS will have more cases with data from multiple time points. Future research should leverage such databases, but also prospective studies of early-stage lung cancer treatment impacts are needed to truly understand and differentiate effects on HRQoL. Further, measurement should extend beyond QoL to also include more specific measures of physical health such as pain and sleep and more specific measures of mental health such as anxiety and depression. It is possible that using more refined and specific tools will elucidate greater treatment differences that will ultimately inform treatment decision-making for early-stage lung cancer patients.
Acknowledgements
This study used the linked Surveillance, Epidemiology, and End Results (SEER)-Medicare Health Outcomes Survey (MHOS) database. The authors acknowledge the efforts of the SEER program of cancer registries, the National Cancer Institute, and the Centers for Medicare and Medicaid Services in creating the SEER-MHOS database.
Funding: This work was supported in part by the National Cancer Institute (P30CA196521).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The Mount Sinai IRB deemed this study exempt, as it used only preexisting, de-identified data. This article does not contain any studies with human participants or animals performed by any of the authors.
References
- Spiro SG, Porter JC. Lung cancer—where are we today? Current advances in staging and nonsurgical treatment. Am J Respir Crit Care Med 2002;166:1166-96. [Crossref] [PubMed]
- Spira A, Ettinger DS. Multidisciplinary management of lung cancer. N Engl J Med 2004;350:379-92. [Crossref] [PubMed]
- Wisnivesky JP, Bonomi M, Henschke C, et al. Radiation therapy for the treatment of unresected stage I-II non-small cell lung cancer. Chest 2005;128:1461-7. [Crossref] [PubMed]
- Timmerman RD, Kavanagh B. Stereotactic Body Radiation Therapy. Curr Probl Cancer 2005;29:120-57. [Crossref] [PubMed]
- Simone CB 2nd, Dorsey JF. Additional data in the debate on stage I non-small cell lung cancer: surgery versus stereotactic ablative radiotherapy. Ann Transl Med 2015;3:172. [PubMed]
- Deng HY, Wang YC, Ni PZ, et al. Radiotherapy, lobectomy or sublobar resection? A meta-analysis of the choices for treating stage I non-small-cell lung cancer. Eur J Cardiothorac Surg 2017;51:203-10. [PubMed]
- Onishi H, Nagata Y, Shirato H, et al. Stereotactic Body Radiotherapy (SBRT, BED ≥ 100 Gy) for Operable Stage I Non-Small Cell Lung Cancer: Is SBRT Comparable to Surgery? Int J Radiat Oncol Biol Phys 2007;69:S86-7. [Crossref]
- Xia T, Li H, Sun Q, et al. Promising clinical outcome of stereotactic body radiation therapy for patients with inoperable Stage I/II non–small-cell lung cancer. Int J Radiat Oncol Biol Phys 2006;66:117-25. [Crossref] [PubMed]
- Crabtree TD, Denlinger CE, Meyers BF, et al. Stereotactic body radiation therapy versus surgical resection for stage I non–small cell lung cancer. J Thorac Cardiovasc Surg 2010;140:377-86. [Crossref] [PubMed]
- Zheng X, Schipper M, Kidwell K, et al. Survival Outcome After Stereotactic Body Radiation Therapy and Surgery for Stage I Non-Small Cell Lung Cancer: A Meta-Analysis. Int J Radiat Oncol Biol Phys 2014;90:603-11. [Crossref] [PubMed]
- Pennathur A, Luketich JD, Abbas G, et al. Radiofrequency ablation for the treatment of stage I non–small cell lung cancer in high-risk patients. J Thorac Cardiovasc Surg 2007;134:857-64. [Crossref] [PubMed]
- Hof H, Herfarth KK, Münter M, et al. Stereotactic single-dose radiotherapy of stage I non–small-cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys 2003;56:335-41. [Crossref] [PubMed]
- Parashar B, Port J, Arora S, et al. Analysis of stereotactic radiation vs. wedge resection vs. wedge resection plus Cesium-131 brachytherapy in early stage lung cancer. Brachytherapy 2015;14:648-54. [Crossref] [PubMed]
- Varlotto J, Fakiris A, Flickinger J, et al. Matched‐pair and propensity score comparisons of outcomes of patients with clinical stage I non–small cell lung cancer treated with resection or stereotactic radiosurgery. Cancer 2013;119:2683-91. [Crossref] [PubMed]
- M.D. Anderson Cancer Center. International Randomized Study to Compare CyberKnife® Stereotactic Radiotherapy With Surgical Resection in Stage I Non-small Cell Lung Cancer 2009;NCT00840749.
- The Netherlands Organisation for Health Research and Development. A Randomized Clinical Trial of Surgery Versus Radiosurgery (Stereotactic Radiotherapy) in Patients With Stage IA NSCLC Who Are Fit to Undergo Primary Resection. NCT00687986, 2008. Available online: https://clinicaltrials.gov/ct2/show/NCT00687986
- Lagerwaard FJ, Aaronson NK, Gundy CM, et al. Patient-Reported Quality of Life After Stereotactic Ablative Radiotherapy for Early-Stage Lung Cancer. J Thorac Oncol 2012;7:1148-54. [Crossref] [PubMed]
- Ricardi U, Filippi AR, Guarneri A, et al. Stereotactic body radiation therapy for early stage non-small cell lung cancer: Results of a prospective trial. Lung Cancer 2010;68:72-7. [Crossref] [PubMed]
- Timmerman R, Mcgarry R, Yiannoutsos C, et al. Excessive Toxicity When Treating Central Tumors in a Phase II Study of Stereotactic Body Radiation Therapy for Medically Inoperable Early-Stage Lung Cancer. J Clin Oncol 2006;24:4833-9. [Crossref] [PubMed]
- Lagerwaard FJ, Haasbeek CJ, Smit EF, et al. Outcomes of risk-adapted fractionated stereotactic radiotherapy for stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2008;70:685-92. [Crossref] [PubMed]
- Schwartz RM, Yip R, Flores RM, et al. The impact of resection method and patient factors on quality of life among stage IA non-small cell lung cancer surgical patients. J Surg Oncol 2017;115:173-80. [Crossref] [PubMed]
- Ostroff JS, Krebs P, Coups EJ, et al. Health-related quality of life among early-stage, non-small cell, lung cancer survivors. Lung Cancer 2011;71:103-8. [Crossref] [PubMed]
- Koczywas M, Williams AC, Cristea M, et al. Longitudinal changes in function, symptom burden, and quality of life in patients with early-stage lung cancer. Ann Surg Oncol 2013;20:1788-97. [Crossref] [PubMed]
- Kenny PM, King MT, Viney RC, et al. Quality of life and survival in the 2 years after surgery for non-small-cell lung cancer. J Clin Oncol 2008;26:233-41. [Crossref] [PubMed]
- Schulte T, Schniewind B, Dohrmann P, et al. The extent of lung parenchyma resection significantly impacts long term quality of life in patients with non-small cell lung cancer. Chest 2009;135:322-9. [Crossref] [PubMed]
- Möller A, Sartipy U. Long-term health-related quality of life following surgery for lung cancer. Eur J Cardiothorac Surg 2012;41:362-7. [Crossref] [PubMed]
- Schulte T, Schniewind B, Walter J, et al. Age-related impairment of quality of life after lung resection for non-small cell lung cancer. Lung Cancer 2010;68:115-20. [Crossref] [PubMed]
- Ilonen IK, Rasanen JV, Knuuttila A, et al. Quality of life following lobectomy or bilobectomy for non-small cell lung cancer, a two year prospective follow-up study. Lung Cancer 2010;70:347-51. [Crossref] [PubMed]
- Vijayvergia N, Shah PC, Denlinger CS. Survivorship in Non-Small Cell Lung Cancer: Challenges Faced and Steps Forward. J Natl Compr Canc Netw 2015;13:1151-61. [Crossref] [PubMed]
- Poghosyan H, Sheldon L, Leveille SG, et al. Health-related quality of life after surgical treatment in patients with non-small cell lung cancer: A systematic review. Lung Cancer 2013;81:11-26. [Crossref] [PubMed]
- Schwartz RM, Yip R, Olkin I, et al. Impact of surgery for stage IA non-small-cell lung cancer on patient quality of life. J Community Support Oncol 2016;14:37-44. [Crossref] [PubMed]
- Chabowski M, Polański J, Jankowska-Polanska B, et al. The acceptance of illness, the intensity of pain and the quality of life in patients with lung cancer. J Thorac Dis 2017;9:2952-8. [Crossref] [PubMed]
- Criswell KR, Owen JE, Thornton AA, et al. Personal responsibility, regret, and medical stigma among individuals living with lung cancer. J Behav Med 2016;39:241-53. [Crossref] [PubMed]
- Lehto RH. Patient views on smoking, lung cancer, and stigma: a focus group perspective. Eur J Oncol Nurs 2014;18:316-22. [Crossref] [PubMed]
- Chapple A, Ziebland S, McPhersen A. Stigma, shame, and blame experienced by patients with lung cancer: qualitative study. BMJ 2004;328:1470. [Crossref] [PubMed]
- Park S, Kang CH, Hwang Y, et al. Risk factors for postoperative anxiety and depression after surgical treatment for lung cancer†. Eur J Cardiothorac Surg 2016;49:e16-21. [Crossref] [PubMed]
- Schwartz RM, Gorbenko K, Kerath SM, et al. Thoracic surgeon and patient focus groups on decision-making in early-stage lung cancer surgery. Future Oncol 2018;14:151-63. [Crossref] [PubMed]
- Ambs A, Warren JL, Bellizzi KM, et al. Overview of the SEER—Medicare Health Outcomes Survey Linked Dataset. Health Care Financ Rev 2008;29:5-21. [PubMed]


