
Clinical implications of the initial SAPS II in veno-arterial extracorporeal oxygenation
Introduction
Veno-arterial (VA) extracorporeal membrane oxygenation (ECMO) is a valid device to for circulatory support in refractory cardiogenic shock. The original cardiopulmonary bypass device was first developed by John Gibbon in the 1950s, which was employed for blood oxygenation during a prolonged cardiac operation only in the operating room (1). The advent of advanced membrane oxygenators circumvented the damage to blood and led to the first successful experience with ECMO in the early 1970s (2). Extracorporeal life support (ECLS) was first employed for the treatment of acute respiratory distress syndrome (ARDS) in neonatal and pediatric populations (3,4). Over the past several decades, ECMO has markedly progressed, and at present it is accepted as an invaluable tool to manage critical patients suffering from serious cardiac and/or respiratory dysfunction refractory to conventional treatment (5). Despite remarkable advances in ECMO devices and general intensive care unit (ICU) management, VA-ECMO requires considerable financial support and specialized expertise resources (6). Furthermore, physicians and centres encounter ethical dilemmas and issues on patient selection, what patient should be managed with ECMO, and cessation timing, when ECMO support should be stopped (7). Because ECMO only can supply supportive therapy rather than disease-modifying treatment, the best outcome on ECMO management could be obtained by the proper patient selection, appropriate ECMO type appliance, and relevant configuration (8). Since the favorable and positive report of EMCO by the conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR) trial, ECMO applications have been geometrically increased and have continued to progress (9). Because cardiac indications for VA-ECMO might greatly differ on physicians and centres, it is significantly important to determine the appropriate VA-ECMO patient. Therefore, VA-ECMO must be restrictively performed on appropriate patients, and identification of pre-ECMO predictors is considered an essential and indispensable process to identify factors predicting in-hospital survival in patients on VA-ECMO. Although many studies reported a great variety of potential risk factors associated with VA-ECMO, there is no accurate measurement tool to predict the survival probability in patients requiring VA-ECMO. We investigated whether the simplified acute physiology score II (SAPS II) score would be a real-time determinant for deciding VA-ECMO initiation and could be a predictor of survival and weaning probability in patients on VA-ECMO.
Methods
Study patients
In a single medical centre, the ECMO program was first initiated from January 2006, and VA-ECMO was carried out on 135 adult patients suffering from primary cardiogenic shock between January 1, 2010 and December 31, 2014. All the patients in this study were aged ≥18 years. To minimize and avoid selection bias, respiratory failure patients undergoing VV-ECMO or another form of ECMO were excluded were excluded from this study. Adult VA ECMO guidelines and indications by Extracorporeal Life Support Organization (ELSO) were strictly applied. Successful VA-ECMO weaning was defined as weaning, followed by stable survival for more than 48 hours. Survival after VA-ECMO was defined as successful weaning and treatment of the underlying medical condition, followed by discharge without any further events. Written informed consent was waived by the Institutional Review Board (IRB) because of the retrospective nature of the study, and ethical approval on this study was done by the IRB of Hallym University Hospital (2013-105).
Data collection
We retrospectively collected data on pre-ECMO patient’s characteristics: age, sex, height, weight, body mass index (BMI), past medical/surgical history, current underlying disease, admission route, information on cardiac arrest, location of cardiac arrest, time of cardiopulmonary resuscitation (CPR), and complications associated with extracorporeal cardiopulmonary resuscitation (ECPR) or CPR. Pre-ECMO data—including laboratory findings, sepsis-related organ failure assessment (SOFA) score, SAPS II, and door-to-ECMO initiation time—and post-ECMO data—ECMO type, mode, duration, anticoagulation, continuous renal replacement therapy (CRRT), intraaortic balloon pump (IABP), transfusion, length of ICU stay and length of hospital stay—were retrospectively evaluated. In this study, the SAPS II was modified to collect the relevant pre-ECMO data from patients just prior to deciding ECMO. The SAPS II is composed of 17 variables: (I) 12 physiological factors, including pulse rate, systolic blood pressure, body temperature, fraction of inspired oxygen (PaO2/FiO2) ratio, urine output, serum sodium/potassium/bicarbonate/bilirubin/urea level, white blood cell count, and Glasgow Coma Scale (GCS) score; (II) age; (III) type of admission such as scheduled surgical, unscheduled surgical, or medical admission; and (IV) 3 variables related to underlying diseases such as acquired immunodeficiency disease, metastatic carcinomas, and haematological malignancies. For sedated patients, the GCS score before sedation was obtained. For the 12 physiological variables, the worst score during the first 24 hours in the ICU was collected. Organ function was assessed using the SOFA score. Acute kidney injury was defined according to the Acute Kidney Injury Network (AKIN) classification and the RIFLE (Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease) (10).
Indications
According to ELSO guidelines, the VA-ECMO application is initially considered at the situation of 50% mortality risk, and then it is indicated at the clinical conditions of 80% mortality risk. One of the most common contraindications is cerebral haemorrhage, because VA-ECMO requires a considerable amount of anticoagulation and may have a great possibility of cerebral haemorrhage aggravation. Another considerable contraindication to VA-ECMO are as follows: (I) irreversible heart that is not a candidate for transplant or unwitnessed cardiac arrest; (II) advanced age; (III) chronic organ dysfunction (emphysema, cirrhosis, and renal failure), severe immunosuppression, or end-stage malignancy; (IV) low compliance (financial, cognitive, psychiatric, or social limitations); and (V) prolonged CPR without adequate tissue perfusion (11,12).
VA ECMO and cannulation
All patients received 3,000 to 5,000 international unit (IU) of intravenous unfractionated heparin just before ECMO cannulation. After confirming activated clotting time (ACT) greater than 180 seconds, peripheral cannulation for ECMO was performed. Any carotid artery cannulation or any central transthoracic approach was not performed. Using the Seldinger technique under the guidance of ultrasound, a venous cannula was placed just below the right atrium via the femoral vein, and an arterial cannula was located in the iliac artery through the femoral artery. Our absolute principle for safe cannulation is the application of ECMO catheter under ultrasonography and fluoroscopy.
VA-ECMO circuit system
The ECMO flow was initially started at 20 mL/kg/min and was increased until sufficient effective flow was reached. The ECMO flow was maintained at 60 to 80 mL/kg/min (range, 3.5–5.0 L/min) with a sweep gas flow of 2 to 6 L/min using 100% oxygen.
VA-ECMO maintenance
All the laboratory test and metabolic panel results were identified to maintain proper organ oxygenation and perfusion. Arterial blood gas analysis (ABGA) and coagulation battery were verified every hour, during the first day of ECMO initiation, and verified at 2-hour intervals from the second day. The oxygenator was set with oxygen flow and sweep air gas according to ABGA results. The ECMO flow was adequately adjusted to maintain at a cardiac index (native plus ECMO) of ≥2.4 L/min/m2, a mean arterial blood pressure of 70-75 mm Hg, and a mixed venous oxygen saturation (SvO2) level of around 70%. During ECMO, all patients were sedated with morphine, midazolam, remifentanil, dexmedetomidine, or fentanyl, and body temperature was maintained between 36 and 37 °C with a membrane oxygenator heat exchanger. Patients who received ECPR and who exhibited a GCS score of <9 were managed with a hypothermia therapy by maintaining their body temperature around 33–34 °C for 24 hours and without administration of sedative drugs. If the patient in hypothermia showed a GCS score ≥9, body temperature was slowly increased at 0.2 °C/hour, and was immediately administered sedation drugs to preserve neurologic function.
Weaning from VA-ECMO
Successful VA ECMO weaning was defined as weaning, followed by stable survival for more than 48 hours. The criteria for weaning included stable vital signs, no definite bleeding foci, SvO2 ≥70%, hematocrit of 30–35%, absence of tamponade or left heart distension, left ventricular ejection fraction ≥35%, and normalized lactic acid level in blood. Furthermore, we identified that all the organ functions were fully recovered and stabilized from underlying problem. The ECMO flow rate was reduced stepwise under continuous monitoring of haemodynamic and respiratory variables.
Statistical analysis
Statistical analyses were performed using MedCalc for Windows version 17.11.5 (MedCalc software, Ostend, Belgium) and the IBM SPSS software (version 22; IBM Corp., Armonk, NY, USA). All data were collected and analysed using Microsoft Excel (Microsoft, Redmond, WA, USA). Continuous variables were evaluated for normality using the Kolmogorov-Smirnov test. Continuous variables showing normality were analysed using Student’s t test and expressed as the arithmetic mean ± standard deviation. Continuous variables not showing normality were analysed using the Mann-Whitney U test and expressed as the median value (25th–75th interquartile). Categorical variables were expressed as frequency distributions and were tested with Fisher’s exact test or Pearson’s Chi-square test. Univariate comparisons between the groups on categorical variables were performed using the Fisher’s exact test or Pearson’s Chi-square test as appropriate. To avoid type 1 error, a Bonferroni post-hoc correction was applied to data that were initially deemed statistically significant by multiplying the number of variables by the P value. Cox proportional hazards model for multivariable analysis was adapted to determine independent predictors of survival and successful ECMO weaning. Overall survival and weaning were calculated according to the Kaplan-Meier method. Independent predictors of overall survival and weaning were also determined by using Cox proportional hazards model. Level of statistical significance was set at a value of 5% (P<0.05). The univariate and multivariate stepwise logistic regression models were used to identify independent factors associated with mortality. Multiple logistic regression analysis using backwards stepwise regression/elimination was used to examine potential independent factors on mortality. Clinically important variables, as well as variables with a level of statistical significance as P<0.20 in univariate logistic regression analysis, were entered as potential candidate variables in the multivariate models to assess viability as independent predictors for ECMO weaning and survival. The results were described as odds ratios (OR) with 95% confidence intervals (CI) and relevant P values. To evaluate the predictive power of the logistic regression model, a receiver operating characteristic (ROC) curves were used and the area under the ROC curves (AUCs) were calculated. Calibration using the Hosmer-Lemeshow goodness-of-fit test was performed to compare the numbers of observed and predicted deaths. Discrimination was evaluated using the AUCs and the AUC was compared by a nonparametric approach. Detailed analysis was also used for further calculation, such as sensitivity, specificity, cutoff values and overall correctness. Cumulative survival curves as a function of time were evaluated by the Kaplan-Meier survival curves using the log-rank test.
Results
A total of 135 adult patients (aged ≥18 years) who underwent VA-ECMO consisted of 41 (30.4%) women and 94 (69.6%) men, with a mean age of 59.4±16.5 years. All the enrolled patients in this study initially presented with primary cardiogenic shock or cardiac arrest. Appropriate indications for VA ECMO on the ELSO guidelines were determined on the basis of final last diagnosis before discharge, not initial diagnosis. Indications for VA ECMO included acute myocardial infarction (n=71), cardiac arrest (n=16), septic shock (n=20), heart failure (n=3), pulmonary thromboembolism (n=6), aortic dissection with acute myocardial infarction (n=1), amniotic fluid embolism (n=1), intracranial hemorrhage during coronary artery bypass graft (n=1), acute respiratory failure (n=1), near-drowning (n=1), myocarditis (n=3), cardiogenic shock combined with intracranial hemorrhage (n=1), anaphylactic shock (n=3), cardiogenic shock developing into septic shock by liver abscess (n=1), acute pancreatitis (n=1), cardiogenic shock by hypothermia (n=1), arrhythmia (n=1), dilated cardiomyopathy (n=1), cardiogenic shock combined with cerebral infarction (n=1), and cardiogenic shock developing into septic shock by empyema (n=1). VA-ECMO was performed on 1 patient who had a chaotic state between cardiac and respiratory failure. VA-ECMO was applied for both cardiac and respiratory support to this difficult patient, and the final diagnosis was sudden respiratory arrest. Detailed patient information about the clinical and demographic characteristics according to in-hospital mortality and VA-ECMO weaning is clearly summarised in Table 1. Of the 135 patients, 35 (25.93%) patients survived and were discharged uneventfully from the hospital, and the remaining 100 (74.07%) unfortunately did not survive. The mean ages of the survivors and the non-survivors were 51.91±17.64 and 62.08±15.40 years, respectively. The survivors consisted of 27 men and 8 women, and the non-survivors consisted of 67 men and 33 women. The average duration of ECMO support in the survivors vs. the non-survivors was 124.09±76.69 vs. 91.02±110.09 h. Compared to the non-survivors, the survivors showed a lower SAPS II (67.77±20.79 vs. 90.29±13.31, P<0.001), a lower SOFA score (12.63±3.49 vs. 15.33±2.28, P<0.001), a lower predicted death rate (71.12±30.51 vs. 94.00±9.36, P<0.001), a higher initial ipH (7.14±0.22 vs. 6.98±0.15, P<0.001), and a lower initial lactate level (7.09±4.93 vs. 12.11±4.84, P<0.001). The average duration of hospital stay in the successful vs. failed weaning groups was 33.43±27.41 vs. 6.35±8.71 days, and the average duration of ICU stay in the successful vs. failed weaning groups was 20.60±16.88 vs. 5.39±5.95 days. The IABP was more frequently used in the non-survivors [18.00% (18/100) vs. 11.43% (4/35), P=0.04]. CPR was more frequently performed on the non-survivors [85.00% (85/100) vs. 48.57% (17/35), P=0.04], and ECPR was more commonly carried out on the non-survivors [75.00% (75/100) vs. 34.29% (12/35), P<0.001]. CPR time was longer in the non-survivors (58.75±41.18 vs. 34.41±21.15, P=0.021). Cardiac arrest in anytime during hospital stay more frequently developed in the non-survivors [85.00% (85/100) vs. 45.71% (16/35), P<0.001], in-hospital cardiac arrest more usually arose in the non-survivors [91.04% (61/67) vs. 8.96% (6/67), P<0.001], whereas out-hospital cardiac arrest more frequently occurred in the survivors [55.88% (19/34) vs. 44.12% (15/34), P<0.001]. Of the 135 patients, 53 (39.26%) had successful weaning, and the remaining 82 (60.74%) did not. The mean ages of the successful and failed weaning groups were 55.43±18.67 and 62.04±14.57 years, respectively. The successful weaning group consisted of 38 men and 15 women, and the failed weaning group consisted of 56 men and 26 women. The average duration of ECMO support in the survivors vs. the non-survivors was 128.13±82.21 vs. 81.15±111.43 h. Additionally, compared to the failed weaning group, the successful weaning group had a lower SAPS II score (74.91±20.81 vs. 90.62±13.56, P<0.001), a lower SOFA score (13.64±3.35 vs. 15.27±2.36, P=0.001), a lower predicted death rate (78.96±27.29 vs. 93.95±9.90, P<0.001), a higher ipH (7.12±0.20 vs. 6.98±0.15, P<0.001), and a lower lactate level (7.37±4.65 vs. 12.44±4.86, P<0.001). CPR was more frequently performed in the failed weaning group [85.37% (70/82) vs. 60.38% (32/53), P=0.002], and ECPR was more commonly carried out in the failed weaning group [74.39% (61/82) vs. 49.06% (26/53), P=0.01]. Cardiac arrest in anytime during hospital stay more frequently developed in the failed weaning group [85.37% (70/82) vs. 58.49% (31/53), P<0.001], in-hospital cardiac arrest more usually arose in the unsuccessful weaning group [59.76% (49/82) vs. 33.96% (18/53), P=0.001], and out-hospital cardiac arrest more frequently occurred in the unsuccessful weaning group [25.61% (21/82) vs. 24.53% (13/53), P=0.001] (Table 1).
Full table
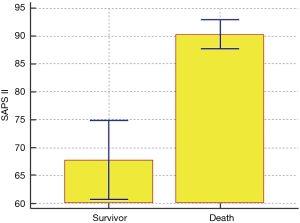
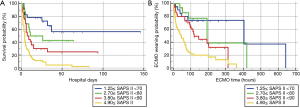
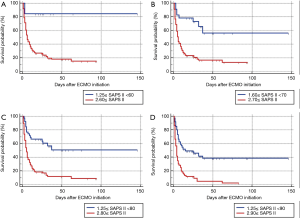
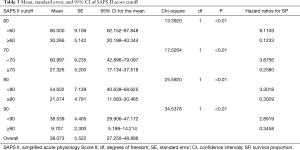
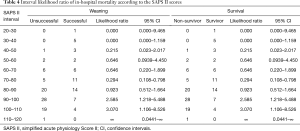
After univariate logistic regression analysis for initial VA-ECMO support parameters, the SAPS II (P<0.001; OR =1.0791; 95% CI, 1.0478–1.1114), CPR (P<0.001; OR =0.1667; 95% CI, 0.0705–0.3941), cardiac arrest (P<0.001; OR =0.1486; 95% CI, 0.0627–0.3520), ipH (P<0.001; OR =0.3149; 95% CI, 0.1887–0.5256), ECPR (P<0.001; OR =0.1739; 95% CI, 0.0757–0.3996), hospital stay (P<0.001; OR =0.8633; 95% CI, 0.8195–0.9094), acute renal failure (P=0.0038; OR =0.2832; 95% CI, 0.1205–0.6655), and age (P=0.0041; OR =1.3048; 95% CI, 1.0878–1.5651) were statistically associated with in-hospital mortality. After multivariate analysis logistic regression analysis for initial VA-ECMO support parameters, the meaningful variables in the final multivariate model were SAPS II, ipH and hospital-stay. The SAPS II (P=0.0389; OR =1.1019; 95% CI, 1.0050–1.2082), ipH (P=0.0452; OR =0.0010; 95% CI, 0.0000–0.8634), and hospital stay (P=0.001; OR =0.8140; 95% CI, 0.7344–0.9023) were statistically associated with in-hospital mortality. The other variables, such as age (P=0.8256), CPR (P=0.7307), ECPR (P=0.5060), acute renal failure (P=0.7018), or cardiac arrest (P=0.9957) were not associated with in-hospital mortality in multivariate analysis logistic regression analysis (Table 2). The SAPS II scores exhibited a significant difference between the survivors and non-survivors (survivor vs. non-survivor; P<0.001; number, 35 vs. 100; arithmetic mean, 67.77 vs. 90.29; 95% CI for the mean, 60.63–74.91 vs. 87.64–92.93; standard deviation, 20.78 vs. 13.30; standard error of the mean, 3.51 vs. 1.33) (Figure 1). To analyse the in-hospital mortality and successful weaning rate, patients in this study were divided into four groups according to SAPS II: those with SAPS II <70 (n=23), those with 70≤ SAPS II <80 (n=16), those with 80≤ SAPS II <90 (n=36), and those with SAPS II ≥90 (n=60). Kaplan-Meier curves for the cumulative survival probability of SAPS II and the cumulative ECMO weaning probability of SAPS II were evaluated. Survival rates were higher in patients with lower SAPS II scores (P<0.001, Chi-square test: χ2=40.4608). Successful weaning rates were noted in patients with lower SAPS II scores (P<0.001, Chi-square test: χ2=34.1365) (Figure 2). In the Kaplan-Meier analysis, the cutoff value SAPS II of 60, 70, 80, and 90 exhibited significant differences in cumulative survival rates (comparison of survival curves by the log rank test: Chi-square test χ2=13.3920, P<0.001 for cutoff 60; Chi-square test χ2=17.5264, P<0.001 for cutoff 70; Chi-square test χ2=25.5920, P<0.001 for cutoff 80; Chi-squared test χ2=34.5378, P<0.001 for cutoff 90) (Figure 3) (Table 3). In our study, interval likelihood ratios for ECMO weaning by SAPS II scores (SAPS II interval, interval likelihood ratio) were summarized in Table 4. Interval likelihood ratios by ROC curve analysis showed that 90–100 and 100–110 were the main cutoff SAPS II scores for increasing VA-ECMO weaning failure (interval likelihood ratios =2.585 vs. 3.070; 95% CI, 1.218–5.488 vs. 1.106–8.526) (Table 4).
Full table
Full table
Full table
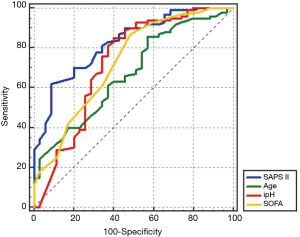
To evaluate the predictive power of the logistic regression model, ROC curves were generated to obtain classification AUCs. The AUCs for the SAPS II score, age, SOFA score, and ipH were assessed by using multiple regression models. The initial SAPS II scores (AUC =0.821) demonstrated significantly better prediction of VA-ECMO mortality than age (AUC =0.697), SOFA score (AUC =0.701), ipH (AUC =0.551), and the other parameters (Figure 4). By the multivariable CoX regression analysis of survival in 135 patients on VA ECMO, only the SAPS II scores proved to have statistical significance. By the multivariable CoX regression analysis of ECMO weaning in 135 patients on VA-ECMO, none of the variables had statistical significance.
Discussion
The hospital mortality rates of patients who underwent ECMO management have been reported to be approximately 60%. Since most of the critically ill patients are treated with VA-ECMO in the ICU, traditional risk scoring systems are applied to patients on VA-ECMO. Pre-existing scoring systems that mainly used in the ICU, including Therapeutic Intervention Scoring System (TISS), Acute Physiology and Chronic Health Evaluation Systems (APACHE I, II, III & IV), Mortality Prediction Model I (MPM I), Mortality Prediction Model II (MPM II), Multiple Organ Dysfunction Score (MODS), Logistic Organ Dysfunction System (LODS), Physiological and Operative Severity Score for the enumeration of Mortality and morbidity (POSSUM) and SOFA, do not meet the requirements, because they demand complicated variables that cannot be obtained in the urgent situation and are difficult to check during the collapsed condition. The appropriate selection of VA-ECMO candidates is especially important for successful treatment, furthermore ECMO requires highly specialized medical staff and equipment. Another important perspective is the emergent nature of ECMO treatment, for which physicians may have a great difficulty in comprehensive discussion and understanding as to whether ECMO should be initiated or not. Unfortunately, there is no universally accepted ECMO-specific risk scoring system to predict early and late outcomes. Surrogates such as the SOFA score and the APACHE score have been used to help assess recovery probability (13). Schmidt et al. (14) reported the Predicting Death for Severe ARDS on VV-ECMO (PRESERVE) scores and Respiratory Extracorporeal membrane oxygenation Survival Prediction (RESP) scores for survival prediction of patients who were treated with ECMO in the ICU, and also reported a new scoring system called “the survival after VA ECMO (SAVE) score” using 12 pre-ECMO parameters. Chen et al. (15) documented that the SAVE score is more acceptable for patients treated with VA ECMO support in the emergency department rather than the PRESERVE or RESP scoring system, and is an independent variable in the Cox proportional hazards regression model. They also reported that a combination of the blood lactic acid level and the SAVE score, termed “the modified SAVE score,” shows more improved outcome prediction for patients receiving VA-ECMO support in the emergency department. In the 2016, the European Society of Cardiology (ESC) guidelines for the diagnosis and treatment of acute and chronic heart failure, mechanical circulatory support in acute heart failure might be applied for management of patients with acute heart failure or cardiogenic shock. Short-term mechanical support systems (MCS), including percutaneous cardiac support devices, ECLS and ECMO may be used for patients with left or biventricular failure until cardiac and other organ function have recovered. On the basis this ESC guidelines, it was strictly recommended that the use of ECLS and ECMO should be restricted to a few days to weeks and the SAVE score should be used to predict survival for patients receiving ECMO for refractory cardiogenic shock. Furthermore, these MCS systems could be used as a bridge to decision making in patients with acute and rapidly deteriorating cardiogenic shock or heart failure to stabilize hemodynamics, recover end-organ function and allow for a complete clinical evaluation for the possibility of either a more durable MCS device or heart transplantation (16).
Actually, the SAPS II scores and the SAVE score share common variables such as age, organ failure, factors related to underlying diseases, heart rate, blood pressure, body temperature, and serum bicarbonate level. Klinzing et al. (17) evaluated that the PRESERVE and RESP scoring systems fail to predict exact mortality for patients under VA-ECMO. The EuroSCORE was actually designed to predict the mortality of patients undergoing cardiac surgery and may have a correlation with the outcomes of postcardiotomy failure patients. The same study assessed patients treated with ECMO for refractory postcardiotomy shock and found that logistic EuroSCORE >20% is associated with mortality (18). Kim et al. (19) reported that the SAPS II score is a powerful predictor of mortality in patients undergoing ECMO in the emergency department. Choi et al. (20) pointed out that the SAPS II score is a potent predictor of mortality in ECMO patients with septic shock and that a cutoff SAPS II score of 80 can be used for ECMO application. Le Gall et al. (21) compared the SAPS II score vs. the mortality rate with good agreement: 29 points vs. 10%, 40 points vs. 25%, 52 points vs. 50%, 64 points vs. 75%, and 77 points vs. 90%. In our study, Survival rates were 80.00% in patients with pre-ECMO SAPS II scores <60 and 30.27% in those with pre-ECMO SAP II scores ≥60; 60.99% in patients with pre-ECMO SAPS II scores <70 and 27.32% in those with pre-ECMO SAP II scores ≥70; 54.63% in patients with pre-ECMO SAPS II scores <80 and 21.074% in those with pre-ECMO SAP II ≥80; and 38.53% in patients with pre-ECMO SAPS II scores <90 and 9.70% in those with pre-ECMO SAP II ≥90. Based on these results, it is conceivable that the pre-ECMO SAPS II score could allow appropriate patients selection for ECMO support and improve outcomes. In our study, the patients with SAPS II scores 90–100 could have 3.733 times higher in-hospital mortality rate. Therefore, the cutoff SAPS II score of 90 can be used as a reference point where VA-ECMO is initiated and can also be used as a reference point where VA-ECMO weaning is initiated. The SAPS II was originally developed for mortality estimation from a large volume of medical and surgical ICU patients in North America and Europe (10). Paradoxically, this SAPS II excluded patients with burn injuries, coronary care unit patients, and/or cardiac surgical patients from the analysis. However, our study adopted the SAPS II for VA-ECMO patients, most of who had coronary artery disease, underwent combined cardiac surgery, and were admitted to the coronary artery or cardiac intensive care unit. The minimal SAPS II score is 0 point, and the maximal SAPS II score is 163 points. Based on calculated SAPS II scores, the correlation between total SAPS II score and in-hospital mortality is statistically analysed. In-hospital mortality is calculated using the following equation: Logit(a) = −7.7631 + 0.0737 × (SAPS II) + 0.9971 × log (SAPS II + 1). Through the SAPS II, we can predict mortality, and the probability P of hospital mortality can be calculated as follows: P = exp (logit)/[1+exp (logit)]. Several reports contended that the SOFA score is a strong predictor for ECMO treatment. Ceriani et al. (22) asserted that the SOFA score is universally applicable in cardiac surgery without requiring specific modifications. Belohlávek et al. (23) showed that patients who expired during ECMO had a higher SOFA score (14.8±1.6 vs. 10.8±1.5; P=0.0065). Wu et al. (24) suggested that a SOFA score of >14 before ECMO support initiation is a good predictor of mortality. In our study, compared to SAPS II, the SOFA score was not a significant predictor for ECMO treatment, but correlation between the SOFA score and the SAPS II can be expressed as follows: SAPS II = 38.724 + (3.156 × SOFA) (P<0.0001; 95% CI, 0.3567−0.6134; r=0.4958). The pre-ECMO SAPS II score might be useful for predicting survival and successful weaning in patients receiving VA-ECMO. The pre-ECMO SAPS II can also be used to decide whether to include cardiogenic shock patients in future randomized studies. In 2015, Schmidt et al. (14) reported that 1,601 (42%) ECMO patients for refractory cardiogenic shock were alive in derivation ELSO cohort (n=3,846) and 108 (67%) patients were alive in validation cohort (n=161) at hospital discharge, and showed superior survival results to our study. Relatively low survival rate (25.93%) in our study compared to the report by Schmidt M et al. might be explained as follow hypothesis: (I) a relatively advanced age (our study group vs. derivation ELSO cohort vs. validation cohort in SAVE, 59.44 vs. 54 vs. 51 years), (II) a great variety of indications for VA ECMO, containing unknown origin cardiac arrest (n=16), septic shock (n=20) and pulmonary thromboembolism (n=6), (III) a high rate of acute renal failure (64/135, 47.4%), and acute or chronic renal failure that was managed under CRRT (97/135, 71.8%), (IV) a high rate of pre-ECMO cardiac arrest (101/135, 74.8%), which means diastolic blood pressure before ECMO was ≤40 mmHg and pulse pressure before ECMO was ≤20 mmHg.
Our study has several limitations. Firstly, this study was conducted at a single institution, which limited the generalisability of the study results. Secondly, our study population was relatively small, had a variety of underlying diseases indicated for VA ECMO including ECPR and a wide variety of VA ECMO subtype including VA, VV-VA, VA-VV, VAV and VA-VAV ECMO. Thirdly, despite a study about VA-ECMO, a relatively small number of patients with refractory septic shock were enrolled in the study. Fourthly, since our study only focused on VA-ECMO, it is difficult to generalise our results to other forms of ECMO such as VV-ECMO. Fifthly, serum biomarkers such as brain natriuretic peptide were not measured in our study, which are known as predictors of outcomes after severe cardiac failure. Further studies are needed to determine whether our results could be accurately applied to such patients. Sixthly, we performed a retrospective analysis, so that additional prospective multicentre studies are needed to confirm our results. Future research should develop more simplified VA-ECMO scoring systems with a larger sample size to accurately predict VA-ECMO mortality. Finally, further studies using various forms of ECMO are warranted. Our study only focused on initial modalities at the decision point of ECMO, so that long-term outcomes were not evaluated.
Conclusions
Despite established ELSO indications for adults VA-ECMO, cardiac indications for ECMO may differ greatly among physicians and centres; therefore, it is extremely important to determine what patient should be treated with VA-ECMO. Although the accurate predictive value of scoring systems will remain one of the biggest challenges to physicians, the SAPS II score can facilitate give objective prognostic information to family members and surrogates and may help physicians increase patient survival rate and avoid a waste of healthcare services.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The present study was approved by the Ethics Committee and the Institutional Review Board (2013-105, Hallym University, Hallym University Chuncheon Sacred Heart Hospital, Republic of Korea). All subjects enrolled in this research have given their informed consent, which has been approved by my institutional committee on human and/or animal research, and this protocol has been found acceptable by them.
References
- Gibbon JH Jr. Application of a mechanical heart and lung apparatus to cardiac surgery. Minn Med 1954;37:171-85. [PubMed]
- Hill JD, O'Brien TG, Murray JJ, et al. Prolonged extracorporeal oxygenation for acute post-traumatic respiratory failure (shock-lung syndrome). Use of the Bramson membrane lung. N Engl J Med 1972;286:629-34. [Crossref] [PubMed]
- Dorson W Jr, Baker E, Cohen ML, et al. A perfusion system for infants. Trans Am Soc Artif Intern Organs 1969;15:155-60. [PubMed]
- White JJ, Andrews HG, Risemberg H, et al. Prolonged respiratory support in newborn infants with a membrane oxygenator. Surgery 1971;70:288-96. [PubMed]
- MacLaren G, Combes A, Bartlett RH. Contemporary extracorporeal membrane oxygenation for adult respiratory failure: life support in the new era. Intensive Care Med 2012;38:210-20. [Crossref] [PubMed]
- Mirabel M, Luyt CE, Leprince P, et al. Outcomes, long-term quality of life, and psychologic assessment of fulminant myocarditis patients rescued by mechanical circulatory support. Crit Care Med 2011;39:1029-35. [Crossref] [PubMed]
- Ramanathan K, Cove ME, Caleb MG, et al. Ethical dilemmas of adult ECMO: emerging conceptual challenges. J Cardiothorac Vasc Anesth 2015;29:229-33. [Crossref] [PubMed]
- Fraser JF, Shekar K, Diab S, et al. ECMO - the clinician’s view. ISBT Sci Ser 2012;7:82-8. [Crossref]
- Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilator support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicenter randomised controlled trial. Lancet 2009;374:1351-63. [Crossref] [PubMed]
- Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 1993;270:2957-63. [Crossref] [PubMed]
- The Extracorporeal Life Support Organization: Extracorporeal Life Support Guidelines, Patient Care Practice Guidelines. Available online: https://www.elso.org/Resources/Guidelines.aspx, accessed on February 01 2016.
- Pujara D, Sandoval E, Simpson L, et al. The State of the Art in Extracorporeal Membrane Oxygenation. Semin Thorac Cardiovasc Surg 2015;27:17-23. [Crossref] [PubMed]
- Peigh G, Cavarocchi N, Keith SW, et al. Simple new risk score model for adult cardiac extracorporeal membrane oxygenation: simple cardiac ECMO score. J Surg Res 2015;198:273-9. [Crossref] [PubMed]
- Schmidt M, Burrell A, Roberts L, et al. Predicting survival after ECMO for refractory cardiogenic shock: the survival after veno-arterial-ECMO (SAVE)-score. Eur Heart J 2015;36:2246-56. [Crossref] [PubMed]
- Chen WC, Huang KY, Yao CW, et al. The modified SAVE score: predicting survival using urgent veno-arterial extracorporeal membrane oxygenation within 24 hours of arrival at the emergency department. Crit Care 2016;20:336. [Crossref] [PubMed]
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891-975. [Crossref] [PubMed]
- Klinzing S, Wenger U, Steiger P, et al. External validation of scores proposed for estimation of survival probability of patients with severe adult respiratory distress syndrome undergoing extracorporeal membrane oxygenation therapy: a retrospective study. Crit Care 2015;19:142. [Crossref] [PubMed]
- Rastan AJ, Dege A, Mohr M, et al. Early and late outcomes of 517 consecutive adult patients treated with extracorporeal membrane oxygenation for refractory postcardiotomy cardiogenic shock. J Thorac Cardiovasc Surg 2010;139:302-11. [Crossref] [PubMed]
- Kim KI, Lee HS, Kim HS, et al. The pre-ECMO Simplified Acute Physiology Score II as a predictor for mortality in patients with initiation ECMO support at the emergency department for acute circulatory and/or respiratory failure: a retrospective study. Scand J Trauma Resusc Emerg Med 2015;23:59. [Crossref] [PubMed]
- Choi MJ, Ha SO, Kim HS, et al. The Simplified Acute Physiology Score II as a Predictor of Mortality in Patients Who Underwent Extracorporeal Membrane Oxygenation for Septic Shock. Ann Thorac Surg 2017;103:1246-53. [Crossref] [PubMed]
- Le Gall JR, Neumann A, Hemery F, et al. Mortality prediction using SAPS II: an update for French intensive care units. Crit Care 2005;9:R645-52. [Crossref] [PubMed]
- Ceriani R, Mazzoni M, Bortone F, et al. Application of the sequential organ failure assessment score to cardiac surgical patients. Chest. 2003;123:1229-39. [Crossref] [PubMed]
- Belohlávek J, Rohn V, Tosovsky J, et al. A review of a newly established ECMO program in a university affiliated cardiac center. J Cardiovasc Surg (Torino) 2011;52:445-51. [PubMed]
- Wu MY, Lin PJ, Tsai FC, et al. Impact of preexisting organ dysfunction on extracorporeal life support for non-postcardiotomy cardiopulmonary failure. Resuscitation 2008;79:54-60. [Crossref] [PubMed]


