
From “high” ZEB1 to “low” B-cell lymphoma 2-interacting mediator of cell death (BIM)—an epithelial-mesenchymal transition (EMT)-associated drug resistance pathway elucidated
A recently published article (1) shows that ZEB1, one of the pivotal transcription factors involved in the induction of epithelial-mesenchymal transition (EMT) in tumor cells, inhibits the expression of the proapoptotic factor B-cell lymphoma (BCL) 2-interacting mediator of cell death (BIM) by binding directly to the BIM promoter and repressing its transcription. This mediates the resistance of epidermal growth factor receptor (EGFR)-mutant lung cancer cells with a mesenchymal phenotype towards EGFR inhibitor (EGFRi)-induced apoptosis. These results are of considerable interest both in terms of our knowledge about the fundamental mechanisms governing EMT-associated effects, as well as their potential therapeutic implications. In order to put the findings in a broader context we will briefly summarize our current knowledge about EMT and its effects.
Tumor cells that undergo EMT loose properties of epithelial cells like apicobasal axis of polarity and cell-cell adhesion and acquire properties of mesenchymal cells like loose three-dimensional organization and, consequently, increased motility (2). There are different settings where EMT can occur. First, in response to stressors from the tumor microenvironment like hypoxia, low pH, immune responses, mechanical stress and antitumor drugs (3). Second, stressor-promoted epigenetic chances that induce heritable effects allowing retention of the mesenchymal state even when the stressors are no longer present (4). Third, stimulus-independent activation of signaling pathways, owing to activating mutations or overexpression of pathway components (5).
A large number of intracellular signaling pathways has been reported being involved in EMT (2). These pathways regulate the expression of a cohort of EMT-promoting transcription factors. These transcription factors can be classified on the basis of their ability to repress E-cadherin directly or indirectly, because loss of E-cadherin expression is considered a crucial event for tumor cells undergoing EMT (6,7). Direct repressors include: zinc finger proteins of the SNAIL superfamily, such as SNAI1/SNAIL, SNAI2/SLUG and SNAI3; zinc finger and E-box binding proteins of the ZEB family, such as ZEB1 and ZEB2; the basic helix-loop-helix factor (bHLH) E47; and the Krüppel-like factor KLF8. Factors such as the bHLH TWIST proteins (TWIST1 and TWIST2), the homeobox proteins goosecoid and SIX1, the bHLH factor E2.2 and the forkhead-box protein FOXC2 repress E-cadherin transcription indirectly.
EMT, however, is not a unidirectional event. In fact, the EMT program is activated reversibly, permitting tumor cells to revert back to more epithelial states via mesenchymal-epithelial transition (8).
From a practical (clinical, prognostic) point-of-view the most important aspect of tumor cell EMT lies in its biological effects. Thus, acquisition of increased motility leads to increased invasiveness, which favors dissemination of tumor cells to distant sites and formation of metastases. In addition, tumor cells become resistant to apoptosis and antitumor drugs, contribute to immunosuppression, and act as cancer stem-like cells (CSCs) (2).
It can be immediately appreciated that these are exactly those biological properties that make tumors resistant to cures and are responsible for the vast majority of deaths among tumor patients. Hence the importance of understanding the basic mechanisms that leads to the biological effects of EMT in order to identify novel therapeutic approaches that address these effects.
Now, where lies the relevance of the findings reported by Song et al. (1)? Beforehand, it is important to point out that, regarding the EMT of tumor cells, we know quite a lot about the signals that induce EMT (hereafter, the “input signals”), but we know relatively little about the signals that lead to the biological effects of EMT (the “output signals”). Are the input signals able to act also as output signals or, stated differently, are the biological effects a direct consequence of EMT or are additional signals required for this purpose? The work of Song et al. (1) gives a significant contribution to our understanding of this aspect.
Taking advantage of models of EMT-induced resistance to EGFRi in EGFR-mutant lung cancers, they observed that mesenchymal EGFR-mutant lung cancer cells are resistant to EGFRi-induced apoptosis via insufficient expression of the proapoptotic protein BIM, thereby preventing cell death despite potent suppression of oncogenic signaling. Insufficient expression of BIM was the consequence of direct binding of the EMT transcription factor ZEB1 to the BIM promoter and, consequently, to the repression of transcription. Increasing free intracellular BIM levels through derepression of its expression by depletion of ZEB1 or treatment with the BCL-2 homology domain 3 (BH3) mimetic ABT-263 led to resensitization of mesenchymal EGFR-mutant cancer cells to EGFRi. The relationship between EMT and loss of BIM was not restricted to EGFR-mutant lung cancers, as it was also observed in KRAS-mutant lung cancers and large datasets, including different cancer subtypes. The authors point out, however, that low BIM is likely only one of multiple mechanisms whereby mesenchymal EGFR-mutant cancers are less sensitive to tyrosine kinase inhibitors like EGFRi.
These results are consistent with the view whereby input signals act also as output signals. Thus, ZEB1, a pivotal EMT transcription factor, is also directly responsible for the induction of resistance to EGFRi in EGFR mutant lung cancer cells. Whether this holds true also for other biological effects of EMT is an open question, but there are indications that this is not necessarily the case. Thus, it has been reported that EMT induces chemoresistance in pancreatic and lung cancer, but is not required for metastasis (9,10). In light of the results of Song et al. (1), this suggests that an input signal is sufficient to induce resistance to drug (EGFRi)-induced apoptosis, but other signals may be necessary to induce metastasis. It has been previously reported that ZEB1 contributes also to metastasis formation in lung cancer models, in particular as regards the establishment of a tumor microenvironment permissive for metastasis formation (11). On these bases one may then conclude that ZEB1 represents a necessary and sufficient condition for the induction of drug resistance, at least as regards EGFRi in EGFR mutant lung cancer cells, but a necessary, yet not sufficient condition for metastatization. In the latter case, one or more supplementary factors may be required in order to achieve sufficiency for metastasis formation and these factors may not be necessarily captured in a single tumor model. Thus, for example, acquisition of an EMT-induced procoagulant state has been reported to contribute to metastasis formation (12).
In addition to this principal finding, this article reports other results of considerable interest. First, it is shown that induction of EMT did not change the ability of EGFRi to down-regulate EGFR phosphorylation or downstream signaling pathways like the phosphoinositide 3-kinase (PI3K)/Akt and extracellular-regulated kinase (ERK) pathways. This is despite the markedly reduced ability of EGFRi to induce apoptosis. This suggests that these signaling pathways are not involved in the resistance to EGFRi in EGFR-mutant lung cancer cells. This is surprising as one of the above pathways, PI3K/Akt, has even been proposed to be a crucial node for EMT induction and point of convergence for other EMT-inducing pathways (13). There are two possibilities to explain these findings. First, signaling pathways like PI3K/Akt may well be involved in the induction of EMT, but may play no further role in the maintenance of a mesenchymal phenotype and one or more of its biological effects (2). Second, a given EMT state is a mosaic-like composition and the assembly of different pieces give rise to different phenotypic pictures along a continuum from a fully epithelial to a fully mesenchymal phenotype (8). The tumor microenvironment would play a key role in the overall composition of the EMT mosaic (14). According to this view it would not come to surprise if different signaling pathways contribute to a given mosaic and, in certain conditions, one or more of the EMT-inducing pathways may play a negligible role, while playing a predominant role in other conditions. Recent results showing that multiple tumor subpopulations associated with different EMT stages display differences in cellular plasticity, invasiveness and metastatic potential, while presenting different transcriptional and epigenetic landscapes appear consistent with this view (14).
Third, further results (1) suggest that mesenchymal EGFR mutant lung cancer cells preexist to and are positively selected by drug (EGFRi) treatment (Figure 1). These cells may then reacquire the ability to grow via a mutational event affecting growth signaling pathways. The possibility that antitumor drugs may positively select preexisting mesenchymal tumor cells has already been proposed (15). Incidentally, the observation of preexisting mesenchymal EGFR mutant lung cancer cells may also explain the apparent paradox that EGFRi have been reported to prevent EMT induction in tumor cells (16). In fact, EGFRi may be able to inhibit the EMT in cells with an epithelial phenotype, but may not be able to do so in cells that have already undergone an EMT, i.e., to reverse an established mesenchymal phenotype. The preexistence of drug-resistant, mesenchymal tumor cells suggests the possibility of a combined therapeutic approach using compounds that act on mesenchymal tumor cells in order to prevent tumor cell regrowth and compounds that act on epithelial tumor cells. This raises the question as to which would be the most appropriate drug class to inhibit and/or deplete mesenchymal tumor cells. Many classes of drugs with the potential to act as inhibitors of EMT induction and/or maintenance of a mesenchymal phenotype have been described (2,8,17). However, if we consider the possibility that different signaling pathways induce different EMT transition states that display different phenotypic profiles, then it may be very difficult to identify a class of drugs active in every setting where EMT occurs. Of course, also in this case one may expect making progress in the identification of biomarkers predicting the contribution of individual signaling pathways in the induction of a given EMT phenotype. On the other hand, the unambiguous identification of mediators of resistance towards EGFRi in EGFR mutant lung cancer cells (ZEB1 and BIM), as described in the article of Song et al. (1), paves the way for the design and testing of compounds that inhibit (ZEB1) or increase the intracellular levels (BIM) of these mediators. In the case of ZEB1, a transcription factor, this appears a challenging but, in perspective, not impossible task. The other possibility, i.e., to increase the intracellular levels of the proapoptotic factor BIM appears more feasible in the shorter term and the authors of this article have shown that this can be achieved in experimental models through the use of the BH3 mimetic ABT-263 that competes with BIM for binding to BCL extra large (xL).
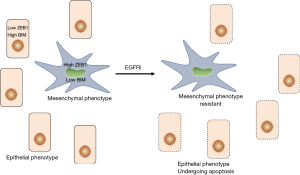
Overall, these are important results which raise several questions. First of all, the present findings bear on EGFR and KRAS mutant lung cancer cells and show that EMT-mediated resistance to EGFRi can be overcome by increasing levels of free intracellular BIM. Then, do these conclusions hold true also for other classes of drugs like chemotherapeutic drugs, radiotherapeutics or even immune checkpoint inhibitors, given that tumor cell-associated immune checkpoint molecules are able to induce EMT (18)? Second, do these conclusions hold true also for other cancer types beyond the herein considered lung (non-small cell lung) cancer? Preliminary answers to these questions can be obtained in suitable animal models using different cancer types and different classes of antitumor drugs. Third, as pointed out by the authors, it is likely that low BIM is only one mechanism of resistance of EGFR-mutant cancers to EGFRi. How, then, can we predict whether a cancer is potentially sensitive to therapeutics that increase free intracellular levels of BIM? The identification of one or more predictable biomarkers would be the obvious solution to this problem.
In conclusion, the work of Song et al. (1) starts elucidating some heretofore unexplained aspects related to EMT-associated biological effects, in particular as regards the resistance of tumor cells to EGFRi in EGFR mutant lung cancer cells. In the models that were investigated this appears to be mediated by the transcription factor ZEB1 leading to down-regulation of the proapoptotic factor BIM. Possibly, these conclusions may also apply to resistance towards other classes of antitumor drugs. Importantly, this article offers also ways for therapeutically addressing this resistance, through enhancing free, intracellular levels of down-regulated BIM. As is normal for an informative article, the results that are reported raise many new questions to be addressed in forthcoming years. In any case, it appears of particular encouragement that some of the findings can be rapidly tested in clinical trials.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Song KA, Niederst MJ, Lochmann TL, et al. Epithelial-to-mesenchymal transition antagonizes response to targeted therapies in lung cancer by suppressing BIM. Clin Cancer Res 2018;24:197-208. [Crossref] [PubMed]
- Marcucci F, Stassi G, De Maria R. Epithelial–mesenchymal transition: a new target in anticancer drug discovery. Nat Rev Drug Discov 2016;15:311-25. [Crossref] [PubMed]
- Marcucci F, Bellone M, Caserta CA, et al. Pushing tumor cells towards a malignant phenotype. Stimuli from the microenvironment, intercellular communications and alternative roads. Int J Cancer 2014;135:1265-76. [Crossref] [PubMed]
- Dumont N, Wilson MB, Crawford YG, et al. Sustained induction of epithelial to mesenchymal transition activates DNA methylation of genes silenced in basal-like breast cancers. Proc Natl Acad Sci U S A 2008;105:14867-72. [Crossref] [PubMed]
- Chung SS, Giehl N, Wu Y, et al. STAT3 activation in HER2-overexpressing breast cancer promotes epithelial-mesenchymal transition and cancer stem cell traits. Int J Oncol 2014;44:403-11. [Crossref] [PubMed]
- Thiery JP, Acloque H, Huang RY, et al. Epithelial-mesenchymal transitions in development and disease. Cell 2009;139:871-90. [Crossref] [PubMed]
- Puisieux A, Brabletz T, Caramel J. Oncogenic roles of EMT-inducing transcription factors. Nat Cell Biol 2014;16:488-94. [Crossref] [PubMed]
- Zhang Y, Weinberg RA. Epithelial-to-mesenchymal transition in cancer: complexity and opportunities. Front Med 2018;12:361-73. [Crossref] [PubMed]
- Zheng X, Carstens JL, Kim J, et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 2015;527:525-30. [Crossref] [PubMed]
- Fischer KR, Durrans A, Lee S, et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 2015;527:472-6. [Crossref] [PubMed]
- Peng DH, Ungewiss C, Tong P, et al. ZEB1 induces LOXL2-mediated collagen stabilization and deposition in the extracellular matrix to drive lung cancer invasion and metastasis. Oncogene 2017;36:1925-38. [Crossref] [PubMed]
- Bourcy M, Suarez-Carmona M, Lambert J, et al. Tissue Factor Induced by Epithelial-Mesenchymal Transition Triggers a Procoagulant State That Drives Metastasis of Circulating Tumor Cells. Cancer Res 2016;76:4270-82. [Crossref] [PubMed]
- Marcucci F, Rumio C. How tumor cells choose between epithelial-mesenchymal transition and autophagy to resist stress—Therapeutic implications. Front Pharmacol 2018;9:714. [Crossref] [PubMed]
- Pastushenko I, Brisebarre A, Sifrim A, et al. Identification of the tumour transition states occurring during EMT. Nature 2018;556:463-8. [Crossref] [PubMed]
- Creighton CJ, Li X, Landis M, et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci U S A 2009;106:13820-5. [Crossref] [PubMed]
- Sato F, Kubota Y, Natsuizaka M, et al. EGFR inhibitors prevent induction of cancer stem-like cells in esophageal squamous cell carcinoma by suppressing epithelial-mesenchymal transition. Cancer Biol Ther 2015;16:933-40. [Crossref] [PubMed]
- Hangauer MJ, Viswanathan VS, Ryan MJ, et al. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature 2017;551:247-50. [PubMed]
- Marcucci F, Rumio C, Corti A. Tumor cell-associated immune checkpoint molecules - Drivers of malignancy and stemness. Biochim Biophys Acta Rev Cancer 2017;1868:571-83. [Crossref] [PubMed]


